Abstract
Despite the high sensitivity of breast magnetic resonance imaging (MRI), pathologic confirmation by biopsy is essential because of limited specificity. MRI-guided biopsy is required in patients with lesions only seen on MRI. We review preprocedural considerations and the technique of MRI-guided biopsy, challenging situations and trouble-shooting, and correlation of radiologic and pathologic findings.
The use of breast magnetic resonance imaging (MRI) is increasing for screening and diagnosis of breast cancer. MRI has greater sensitivity than other imaging modalities for the screening of women at higher risk of breast cancer, with more than half of lesions only detected on MRI (12). MRI can identify primary cancer in suspected occult breast cancer patients (3). In addition, breast MRI most accurately depicts the extent of breast cancer (456). Although the clinical relevance of additional lesions is still controversial, preoperative MRI identifies more synchronous, ipsilateral or contralateral breast cancers than mammography and ultrasound (789).
However, breast MRI has limited specificity (10). If a suspicious lesion is detected, biopsy is mandatory to avoid unnecessary surgery. In addition, biopsy under MRI guidance is required for suspected occult lesions on mammography or ultrasound. The American College of Radiology requires performance of MRI-guided intervention or contact with available referral site when performing breast MRI (11). The MRI guideline of the European Society of Breast Imaging also emphasizes the necessity of offering MRI-guided intervention at a site performing breast MRI (12). The number of medical centers providing breast MRI is growing in Korea, and more MRI-guided breast biopsies are required. However, few reports describe MRI-guided breast biopsy in Korea (1314). In this article, we describe preprocedural considerations and technique, and correlation of radiologic and pathologic findings in MRI-guided breast biopsy.
Second-look studies should be considered for all women with suspicious lesions on MRI. If the lesion is delineated with mammography or ultrasound, biopsy under imaging guidance using these modalities is preferred. MRI-guided biopsy is more expensive and difficult for the patient. However, the reported correlation rate of second-look ultrasound ranges from 23–57%; MRI-only lesions require an MRI-guided biopsy (15161718).
Informed consent should be obtained before the biopsy. Patients with a known contraindication for MRI or gadolinium administration should not have an MRI-guided biopsy. Patients with allergy to gadolinium or local anesthetics are also not suitable. Bleeding risk due to use of aspirin or anticoagulants or an underlying coagulation disorder is a relative contraindication for biopsy, requiring careful consideration of the risks and benefits. The patient should be able to remain prone during the biopsy for a minimum of 60 minutes. The possibility of a nonvisualized target lesion should be discussed with the patient. Even with successful visualization, the cancellation of a biopsy is sometimes necessary because of unforeseen safety issues. Complications such as hematoma, infection, and skin injury should also be discussed with the patient. For patients with breast implants, rupture is a possible complication of the biopsy.
Table 1 summarizes the biopsy procedure.
The patient lies in a prone position, and a dedicated interventional breast coil is used. Either a grid or a pillar and post system is generally used. We focus on the grid system, which is most widely used (Fig. 1). The breast within the coil is compressed by the grid. Compression pressure is adjusted for stable immobilization while preserving blood flow. The approach to a posterior mass is often difficult, requiring that sufficient breast tissue be contained in the grid to maintain distance from the chest wall. Fixing a fiducial marker to the grid enhances target localization.
MRI-guided vacuum assisted biopsy (VAB) has advantages over standard core biopsy. The larger core size results in decreased sampling error and compensates for tissue shifting during needle placement (19). The European consensus group recommend a minimum 11-gauge probe size (20). The MRI-guided biopsy kit consists of an introducer stylet, obturator, introducer sheath, and needle guide. The needle guide, a cube-shaped plastic block with multiple holes for the biopsy device (Fig. 2), maintains the VAB device perpendicular to the grid.
Fig. 3 shows the MRI-guided biopsy sequence. A precontrast T1-weighted fat-saturation sequence is obtained to determine whether the target lesion is within the grid. If the target lesion is too small for detection on the precontrast image, anatomic landmarks are helpful for identification. If the precontrast image indicates that the lesion is inaccessible, the patient's position should be adjusted. After planning the proper approach route, postcontrast T1-weighted fat-saturation images are obtained. The thickness and in-plane resolution, which are similar to that of diagnostic imaging for accurate needle positioning, make localization easier. Sagittal and axial plane images are obtained. Alternatively, axial or sagittal plane images with perpendicular reconstruction can be used for lesion localization depending on the physician's preference. The entry site on the grid and depth from the skin to the lesion are measured using the fiducial marker as a reference. A worksheet provided by the manufacturer is helpful in manual localization (Fig. 4). The thickness of the needle guide should be included in the calculation. A computer-assisted diagnostic system that improves accuracy and speed of the procedure is also commercially available. About 8–13% of MRIguided biopsy target lesions are not visible at the time of biopsy (212223). If the target is not visible on the first postcontrast image, it is sometimes identified on a delayed image. Overpressure by the grid may interfere with breast perfusion and should be checked. A subtraction image also aids in lesion identification (Fig. 5). If the target lesion is still not visible, short-term followup is recommended at about 6 months.
After standard skin preparation, local anesthesia is administered. A small nick in the skin facilitates smooth entry by the VAB device. The introducer stylet within its sheath is inserted through the needle guide to the measured depth. A twisting motion is helpful to avoid skin tenting and tissue displacement. The stylet is removed and replaced by the obturator. T1-weighted fat-saturation images are then obtained to confirm the depth and position of the introducer (2425). In the case of insufficient depth, the introducer sheath can be advanced after reinsertion of the stylet; if advanced past the target lesion, the introducer is gradually withdrawn. In general, the optimal position is in the center of the target. Directional sampling can be performed with a peripheral location of the needle. When the introducer position is verified as correct, the obturator is exchanged with a VAB device. The European consensus group recommends no less than 24 samples for an 11-gauge or equivalent volume if a larger probe is used (20). Liberman (26) reported that an 11-gauge VAB device collects 100 mg, and a 9-gauge VAB device collects 200 mg. T1-weighted fat-saturation images are obtained immediately after the biopsy to evaluate adequacy. Image assessment can be difficult due to contrast washout, background enhancement, hemorrhage, and air, but careful review using anatomic landmarks improves the evaluation accuracy. An additional biopsy can be performed if the target sample is insufficient. Once the target sampling is acceptable, marker clip should be placed through the introducer sheath. A post-clip-insertion image may be obtained with MRI or mammography. The importance of marker clip insertion should never be underestimated. The marker clip facilitates mammographic or ultrasound-guided localization in place of MRI-guided localization for subsequent excisional biopsy. Moreover, if the entire target lesion is removed by VAB, the marker clip is the only way to identify the biopsy site. The breast is compressed for at least 15 minutes after the biopsy.
Major complications requiring surgical intervention seldom occur with MRI-guided biopsy. Complication rates are less than 5%. Bleeding and hematoma formation are most common, and can be controlled by compression. Other rare complications are skin laceration, vasovagal syncope, and infection. Termination of the procedure due to a complication is rare (1927282930313233343536).
Targeting deep-seated lesions is prone to chest wall injury. To avoid this, traction on the breast tissue as much as possible, coil padding removal, and biopsy toward the anterior side are some solutions (33). A posteromedial target location is the most difficult to access. If the patient is small, a lateral approach from the contralateral opening of the breast coil can make a deep posterior location more accessible (Fig. 6). A thin breast is another challenge for MRI-guided biopsy. A generous amount of anesthetic agent helps to increase breast thickness, and a reverse compression paddle is also useful (2437). VAB devices with smaller apertures or blunt tips can be used for targets in thin breasts and also those near the skin (Table 2).
As with all other image-guided biopsy techniques, MRI-guided biopsy results should be evaluated for radiologic-pathologic concordance. MRI-guided biopsy has no corresponding evaluation method such as specimen imaging in stereotactic biopsy or real-time monitoring in ultrasound-guided biopsy; therefore, radiologic-pathologic concordance requires caution. The positive predictive value of a lesion detected by MRI with subsequent MRI-guided biopsy is 16–61%; a radiologist should be aware that for radiologic-pathologic correlation, the positive predictive value is affected by the prevalence of breast cancer in a patient population (19303336383940). A six-month follow-up is recommended for a benign concordant biopsy result (41). The rate of radiology-pathology discordance is not high (0% to 10.7%); but the mean proportion of malignancies in discordant cases is 37.5%, and surgical excision is recommended for discordant lesions (Table 3) (19293034363839).
The atypical ductal hyperplasia (ADH) upgrade rate at surgery is reportedly 25–38% (193038404243). In ductal carcinoma in situ (DCIS), the upgrade rate ranges from 5–24% (19303844). The underestimation rates for ADH and DCIS on MRI-guided biopsy are slightly higher than those for stereotactic biopsy (21% and 11%, respectively) (4546) or ultrasoundguided biopsy (23.3% and 13.8%, respectively) (47). Atypical lobular hyperplasia and lobular carcinoma in situ also have a high upgrade rate (27%) (40). Despite a limited number of studies in the underestimation rate for other high-risk lesions such as radial scars and papillomas, surgical excision for all such lesions using MRI-guided biopsy is recommended (303840).
Breast MRI is an important screening and diagnostic tool, but the limited specificity requires biopsy confirmation. MRIonly lesions that are occult on mammography and ultrasound require routine evaluation by MRI-guided biopsy. Radiologists who perform the procedure understand best the indications, preprocedural considerations, imaging protocols, biopsy techniques, and possible complications of MRI-guided VAB. Patients should be informed about the demanding nature of the procedure due to prolonged immobilization, the possibility of cancellation of the procedure, and the need for imaging follow-up despite a benign biopsy result. Appropriate patient management based on radiologic-pathologic correlation is emphasized.
Figures and Tables
Fig. 2
MRI-guided biopsy kit contains introducer stylet, obturator, introducer sheath, and needle guide.
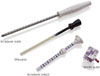
Fig. 3
MRI-guided VAB procedure.
After localizing image (A), precontrast images with fiducial marker (arrow) (B) are obtained. Sagittal and axial postcontrast images (C, D) are obtained to identify target location (arrow).
VAB = vacuum assisted biopsy
After location of introducer sheath and obturator, sagittal and axial images (E, F) are obtained to confirm the position before lesion sampling. The target lesion (arrowhead) and obturator (arrow) are well demonstrated in these images. After tissue sampling, additional sagittal and axial images (G, H) are obtained to confirm adequate biopsy location and marker (arrow) placement. The target lesion was confirmed as ductal carcinoma in situ.
VAB = vacuum assisted biopsy
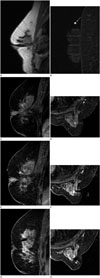
Fig. 5
A 35-year-old woman with ipsilateral breast cancer surgery 9 months previously.
Postoperative MRI shows a new, round, fast and washout-enhancing mass (A). The patient underwent MRI-guided biopsy. The target lesion is not identified on the postcontrast sagittal image (B). On the subtraction image (C), a subtle enhancing mass is well-delineated (arrow). Repeat MRI (D, E) shows introducer sheath with obturator (arrowhead) in correct position, with the tip at the target lesion (arrow). The enhancing mass was pathologically confirmed as reactive hyperplasia in intramammary lymph node.
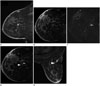
Table 1
Summary of Breast Biopsy MRI Procedure

Table 2
Challenging Situations and Troubleshooting

Table 3
Radiology-Pathology Discordance and Malignancy Rate in Discordant Cases

| Author | Biopsy Device | Number of Lesions (No. of Patients) | Benign Biopsy Result | Discordance | Discordance Rate of Total Biopsy (%) | Malignancy Rate of Discordant Cases (%) |
|---|---|---|---|---|---|---|
| Perlet et al.19 | 11 G VAB | 517 (N/A) | 362 | 0 | 0 | N/A |
| Gebauer et al.29 | 10 G VAB | 42 (32) | 28 | 1 | 2.4 | 100 (1/1) |
| Han et al.30 | 9 or 10 G VAB | 150 (134) | 90 | 1 | 0.7 | 0 (0/1) |
| Malhaire et al.34 | 10 G VAB | 72 (72) | 29 | 3 | 10.3 | 66.7 (2/3) |
| Noroozian et al.36 | 9 G VAB | 75 (75) | 56 | 8 | 10.7 | 0 (0/8) |
| Orel et al.38 | 9 G VAB | 85 (75) | 15 | 2 | 2.4 | 100 (2/2) |
| Rauch et al.39 | 9 G VAB | 218 (197) | 133 | 1 | 0.5 | 100 (1/1) |
References
1. Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010; 28:1450–1457.
2. Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012; 307:1394–1404.
3. Morris EA, Schwartz LH, Dershaw DD, van Zee KJ, Abramson AF, Liberman L. MR imaging of the breast in patients with occult primary breast carcinoma. Radiology. 1997; 205:437–440.
4. Boetes C, Mus RD, Holland R, Barentsz JO, Strijk SP, Wobbes T, et al. Breast tumors: comparative accuracy of MR imaging relative to mammography and US for demonstrating extent. Radiology. 1995; 197:743–747.
5. Gavenonis SC, Roth SO. Role of magnetic resonance imaging in evaluating the extent of disease. Magn Reson Imaging Clin N Am. 2010; 18:199–206. vii–viii.
6. Mann RM. The effectiveness of MR imaging in the assessment of invasive lobular carcinoma of the breast. Magn Reson Imaging Clin N Am. 2010; 18:259–276. ix
7. Pediconi F, Catalano C, Roselli A, Padula S, Altomari F, Moriconi E, et al. Contrast-enhanced MR mammography for evaluation of the contralateral breast in patients with diagnosed unilateral breast cancer or high-risk lesions. Radiology. 2007; 243:670–680.
8. Wang SY, Long JB, Killelea BK, Evans SB, Roberts KB, Silber A, et al. Preoperative breast magnetic resonance imaging and contralateral breast cancer occurrence among older women with breast cancer. J Clin Oncol. 2016; 34:321–328.
9. Iacconi C, Galman L, Zheng J, Sacchini V, Sutton EJ, Dershaw D, et al. Multicentric cancer detected at breast MR imaging and not at mammography: important or not? Radiology. 2016; 279:378–384.
10. Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008; 246:116–124.
11. American College of Radiology. ACR practice parameter for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. 2014. Accessed May 4, 2016. Available at: http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/MRI_Breast.pdf.
12. Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008; 18:1307–1318.
13. Jung HN, Han BK, Ko EY, Shin JH. Initial experience with magnetic resonance-guided vacuum-assisted biopsy in Korean women with breast cancer. J Breast Cancer. 2014; 17:270–278.
14. Choi HY, Kim SM, Jang M, Yun BL, Kim SW, Kang E, et al. MRI-guided intervention for breast lesions using the freehand technique in a 3.0-T closed-bore MRI scanner: feasibility and initial results. Korean J Radiol. 2013; 14:171–178.
15. Abe H, Schmidt RA, Shah RN, Shimauchi A, Kulkarni K, Sennett CA, et al. MR-directed ("Second-Look") ultrasound examination for breast lesions detected initially on MRI: MR and sonographic findings. AJR Am J Roentgenol. 2010; 194:370–377.
16. LaTrenta LR, Menell JH, Morris EA, Abramson AF, Dershaw DD, Liberman L. Breast lesions detected with MR imaging: utility and histopathologic importance of identification with US. Radiology. 2003; 227:856–861.
17. Meissnitzer M, Dershaw DD, Lee CH, Morris EA. Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended. AJR Am J Roentgenol. 2009; 193:1025–1029.
18. Lee SH, Kim SM, Jang M, Yun BL, Kang E, Kim SW, et al. Role of second-look ultrasound examinations for MR-detected lesions in patients with breast cancer. Ultraschall Med. 2015; 36:140–148.
19. Perlet C, Heywang-Kobrunner SH, Heinig A, Sittek H, Casselman J, Anderson I, et al. Magnetic resonance-guided, vacuum-assisted breast biopsy: results from a European multicenter study of 538 lesions. Cancer. 2006; 106:982–990.
20. Heywang-Köbrunner SH, Sinnatamby R, Lebeau A, Lebrecht A, Britton PD, Schreer I. Consensus Group. Interdisciplinary consensus on the uses and technique of MR-guided vacuum-assisted breast biopsy (VAB): results of a European consensus meeting. Eur J Radiol. 2009; 72:289–294.
21. Hefler L, Casselman J, Amaya B, Heinig A, Alberich T, Koelbl H, et al. Follow-up of breast lesions detected by MRI not biopsied due to absent enhancement of contrast medium. Eur Radiol. 2003; 13:344–346.
22. Brennan SB, Sung JS, Dershaw DD, Liberman L, Morris EA. Cancellation of MR imaging-guided breast biopsy due to lesion nonvisualization: frequency and follow-up. Radiology. 2011; 261:92–99.
23. Perlet C, Heinig A, Prat X, Casselman J, Baath L, Sittek H, et al. Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur Radiol. 2002; 12:1463–1470.
24. Liberman L, Morris EA, Dershaw DD, Thornton CM, Van Zee KJ, Tan LK. Fast MRI-guided vacuum-assisted breast biopsy: initial experience. AJR Am J Roentgenol. 2003; 181:1283–1293.
25. Price ER. Magnetic resonance imaging-guided biopsy of the breast: fundamentals and finer points. Magn Reson Imaging Clin N Am. 2013; 21:571–581.
26. Liberman L. Percutaneous image-guided core breast biopsy. Radiol Clin North Am. 2002; 40:483–500. vi
27. Bahrs SD, Hattermann V, Preibsch H, Hahn M, Staebler A, Claussen CD, et al. MR imaging-guided vacuum-assisted breast biopsy: reduction of false-negative biopsies by short-term control MRI 24-48 h after biopsy. Clin Radiol. 2014; 69:695–702.
28. Crystal P, Sadaf A, Bukhanov K, McCready D, O'alley F, Helbich TH. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: can underestimation be predicted? Eur Radiol. 2011; 21:582–589.
29. Gebauer B, Bostanjoglo M, Moesta KT, Schneider W, Schlag PM, Felix R. Magnetic resonance-guided biopsy of suspicious breast lesions with a handheld vacuum biopsy device. Acta Radiol. 2006; 47:907–913.
30. Han BK, Schnall MD, Orel SG, Rosen M. Outcome of MRIguided breast biopsy. AJR Am J Roentgenol. 2008; 191:1798–1804.
31. Hauth EA, Jaeger HJ, Lubnau J, Maderwald S, Otterbach F, Kimmig R, et al. MR-guided vacuum-assisted breast biopsy with a handheld biopsy system: clinical experience and results in postinterventional MR mammography after 24 h. Eur Radiol. 2008; 18:168–176.
32. Kuhl CK, Morakkabati N, Leutner CC, Schmiedel A, Wardelmann E, Schild HH. MR imaging--guided large-core (14-gauge) needle biopsy of small lesions visible at breast MR imaging alone. Radiology. 2001; 220:31–39.
33. Liberman L, Bracero N, Morris E, Thornton C, Dershaw DD. MRI-guided 9-gauge vacuum-assisted breast biopsy: initial clinical experience. AJR Am J Roentgenol. 2005; 185:183–193.
34. Malhaire C, El Khoury C, Thibault F, Athanasiou A, Petrow P, Ollivier L, et al. Vacuum-assisted biopsies under MR guidance: results of 72 procedures. Eur Radiol. 2010; 20:1554–1562.
35. Meeuwis C, Veltman J, van Hall HN, Mus RD, Boetes C, Barentsz JO, et al. MR-guided breast biopsy at 3T: diagnostic yield of large core needle biopsy compared with vacuum-assisted biopsy. Eur Radiol. 2012; 22:341–349.
36. Noroozian M, Gombos EC, Chikarmane S, Georgian-Smith D, Raza S, Denison CM, et al. Factors that impact the duration of MRI-guided core needle biopsy. AJR Am J Roentgenol. 2010; 194:W150–W157.
37. Jackman RJ, Marzoni FA Jr. Stereotactic histologic biopsy with patients prone: technical feasibility in 98% of mammographically detected lesions. AJR Am J Roentgenol. 2003; 180:785–794.
38. Orel SG, Rosen M, Mies C, Schnall MD. MR imaging-guided 9-gauge vacuum-assisted core-needle breast biopsy: initial experience. Radiology. 2006; 238:54–61.
39. Rauch GM, Dogan BE, Smith TB, Liu P, Yang WT. Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. AJR Am J Roentgenol. 2012; 198:292–299.
40. Heller SL, Elias K, Gupta A, Greenwood HI, Mercado CL, Moy L. Outcome of high-risk lesions at MRI-guided 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2014; 202:237–245.
41. Li J, Dershaw DD, Lee CH, Kaplan J, Morris EA. MRI followup after concordant, histologically benign diagnosis of breast lesions sampled by MRI-guided biopsy. AJR Am J Roentgenol. 2009; 193:850–855.
42. Liberman L, Holland AE, Marjan D, Murray MP, Bartella L, Morris EA, et al. Underestimation of atypical ductal hyperplasia at MRI-guided 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007; 188:684–690.
43. Strigel RM, Eby PR, Demartini WB, Gutierrez RL, Allison KH, Peacock S, et al. Frequency, upgrade rates, and characteristics of high-risk lesions initially identified with breast MRI. AJR Am J Roentgenol. 2010; 195:792–798.
44. Lee JM, Kaplan JB, Murray MP, Mazur-Grbec M, Tadic T, Stimac D, et al. Underestimation of DCIS at MRI-guided vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007; 189:468–474.
45. Eby PR, Ochsner JE, DeMartini WB, Allison KH, Peacock S, Lehman CD. Frequency and upgrade rates of atypical ductal hyperplasia diagnosed at stereotactic vacuum-assisted breast biopsy: 9-versus 11-gauge. AJR Am J Roentgenol. 2009; 192:229–234.
46. Jackman RJ, Burbank F, Parker SH, Evans WP 3rd, Lechner MC, Richardson TR, et al. Stereotactic breast biopsy of nonpalpable lesions: determinants of ductal carcinoma in situ underestimation rates. Radiology. 2001; 218:497–502.
47. Lee SH, Kim EK, Kim MJ, Moon HJ, Yoon JH. Vacuum-assisted breast biopsy under ultrasonographic guidance: analysis of a 10-year experience. Ultrasonography. 2014; 33:259–266.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download