Abstract
Mesenteric fibromatosis is a rare benign fibroblastic tumor; moreover, cases that occur in the mesocolon are even rarer. In some cases, mesenteric fibromatosis is difficult to differentiate from a malignant tumor that shows an infiltrative growth pattern or forms intussusception similar to lymphoma or adenocarcinoma. In this study, we reported a case of mesenteric fibromatosis represented as a colo-colic type intussusception adjacent to the ascending colon mimicking malignant tumors such as lymphoma or adenocarcinoma.
Mesenteric fibromatosis is a rare benign fibroblastic tumor that accounts for only about 0.03% of all neoplasms and 8% of all desmoid tumors (1). Most cases of mesenteric fibromatosis occur in the small bowel mesentery (2). Most commonly, the tumor presents as a local invasion and recurrence, without metastasis. Although mesenteric fibromatosis has been reported in various sites, its presentation in the mesocolon is uncommon. Furthermore, the differential diagnosis from other mimicking tumors, such as lymphoma or adenocarcinoma in the colon, can be difficult.
In this study, we reported a case of a 47-year-old patient with mesenteric fibromatosis represented as the colo-colic type intussusception adjacent to the ascending colon that mimics malignant tumor, such as adenocarcinoma or lymphoma; furthermore, we discussed relevant radiological and pathologic findings that can be helpful for the differential diagnosis.
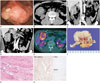
A 47-year-old male presented at our hospital with complaints of abdominal pain for 3 months prior. He had no underlying disease or any history of surgical operation. There were no specific findings on physical examination and the results of the routine laboratory test were normal. At the 2nd day of admission, he underwent the colonoscopy and there was a huge fungating mass with ulceration in the mid ascending colon. The mass was too hard for the biopsy needle to pass through easily; therefore, no specific diagnosis was made by the endoscopic biopsy (Fig. 1A). The abdomen-pelvic CT with contrast enhancement was performed on the following day for the characterization of the tumor. The pre-contrast CT scan showed a well-defined bulky mass at the right upper abdomen (Fig. 1B). This mass measured 7.2 cm in the largest diameter and revealed heterogeneous low density with no hemorrhage or calcifications. On the contrast-enhanced images, the mass presented as heterogeneous, relatively less enhanced and causing colo-colic intussusceptions (Fig. 1C, D). Colonoscopic findings of endoluminal leading point showed a lobulating contour, but an overlying inner layer suggested a well-preserved mucosa (Fig. 1C, D). In addition to the absence of bowel obstruction and other extra-intestinal manifestations, there was no pericolic fat infiltration or significantly enlarged lymph nodes adjacent to the lesion. Under the impression of a large bowel lymphoma causing colo-colic intussusception, 18F-fluorodeoxyglucose (FDG) positron emission tomography/CT (PET/CT) was performed to evaluate systemic involvement on the 4th day of admission. On the 18F-FDG PET/CT, the mass showed a high FDG uptake (maximum standardized uptake value = 5.8); other FDG uptake lesions were not detected (Fig. 1E). The preoperative impression was that of a lymphoma or an adenocarcinoma; therefore, the patient underwent laparoscopic right hemicolectomy.
Grossly, a well-defined solid mass measuring 7.5 × 7 cm abutting the ascending colon was observed. Overlying mucosa showed no abnormality. The cut surface of the mass showed a whitish color and contained whirling pattern fibers without necrosis or hemorrhage (Fig. 1F). On microscopic evaluation, the tumor invaded the muscle layer of the colonic wall. The tumor was composed of bundles of short spindle cells and collagen fiber infiltrating from serosa to the muscle layer with minimal inflammation (Fig. 1G). On immunohistochemistry stain, tumor cells were positive for beta catenin and negative for C-kit and CD-34 (Fig. 1H). Based on the immunohistochemistry results, this tumor was confirmed as mesenteric fibromatosis, benign fibroblastic proliferation.
The patients was discharged without complication and there was no recurrence during the 1 year follow-up after surgery.
Mesenteric fibromatosis is the most frequent form of intraabdominal desmoids tumor (1). Thirteen percent of patients with mesenteric fibromatosis have familial adenomatous polyposis (FAP), specifically, the Gardner syndrome variant of FAP. Prior abdominal surgery is an important risk factor in patients with FAP (2).
The CT findings of mesenteric fibromatosis can be generally categorized into the well-circumscribed form or the infiltrative form. The well-circumscribed form has a well-defined margin and shows homogeneous enhancement with similar attenuation relative to muscles (34). It is also often characterized by considerable size, which envelops the adjacent structures, especially, the small-bowel loops. In the well-circumscribed form, as in the current case, the tumor can be confused with lymphoma. The colonic lymphoma usually appears as a polypoid mass that has a circumferential wall thickening and a homogeneous enhancement (5), not very different from benign features of mesenteric fibromatosis. However, lymphoma usually has lymphadenopathy and infiltrates to other abdominal organs, such as liver and spleen, which is absent in the case of mesenteric fibromatosis (5).
The infiltrative form shows aggressive features such as an ill-defined or irregular margin and a rapid growth pattern that looks malignant (4). The imaging findings of this form are similar to those of adenocarcinoma. However, adenocarcinoma often shows metastatic lymphadenopathy and more intense contrast enhancement (6); furthermore, another key distinguishing point from mesenteric fibromatosis is the pattern in the bowel wall invasion. A desmoid tumor arises from extra-intestinal mesenteric structures such as the mesocolon, so the invasion of the muscle or the mucosal layer occurs later than in adenocarcinoma (2).
18F-FDG PET/CT is a good tool to evaluate the malignant potential of tumors based on FDG uptake (7). However, it is not the absolute cancer-specific modality (8). It is not clear why mesenteric fibromatosis could reveal false-positive imaging on 18F-FDG PET/CT like lymphoma or adenocarcinoma (78). Metser and Even-Sapir discussed many similar cases of benign lesions with high FDG uptake and their possible mechanisms. They suggested possible causes of false positive findings of 18F-FDG PET/CT like that vigorous smooth muscle activity or metabolically active mucosa (7).
In the current case, the tumor was composed of short spindle cells and collagen fiber, but there was a lack of inflammatory cells. The absence of inflammatory cells distinguishes mesen teric fibromatosis from inflammatory myofibroblastic tumor. In addition, positive immunohistochemistry finding for beta catenin supports the diagnosis of mesenteric fibromatosis (19). The high cellularity of the mass composed of fibrotic proliferation may be related to the hardened feature.
Mesenteric fibromatosis is a predisposing factor of intussusception (2). The mechanism of intussusception is believed to involve the bowel or lumen that act as irritant and provoke abnormal peristaltic movement, which may lead to the invagination of one bowel segment into the adjacent segment. Mesenteric fibromatosis also acts as lead point for an intussusception as other masses (10). Mesenteric fibromatosis can cause not only intussusception but also a small bowel obstruction or fistula formation (2). Therefore, the surgical resection is the recommended treatment option. However, the local recurrence is a major issue of mesenteric fibromatosis, even after complete surgical resection. If the resection margin of the tumor is positive or the patient shows functional loss on follow-up, an additional therapy, such as external radiation therapy, non-steroidal anti-inflammatory drug, antiestrogen therapy, or chemotherapy, can be considered (1).
In conclusion, in this study, we described a rare case of mesenteric fibromatosis involving the mesocolon, which mimicked other malignancies like lymphoma or adenocarcinoma. Such a case can be difficult to diagnose in advance of a surgical resection.
Figures and Tables
Fig. 1
A 47-year-old man presented with mesenteric fibromatosis at the ascending mesocolon forming colo-colic intussusception and mimicking malignant tumors.
A. Colonoscopy shows a very large fungating mass with ulceration in the mid ascending colon. The biopsy needle cannot pass through the mass due to its hard-rubber like texture.
B. The axial pre-contrast CT image reveals an approximately 7.2 cm well-defined mass at the right upper quadrant abdomen, which shows some high density area (*).
C, D. The coronal reformatted contrast-enhanced CT image in the portal venous phase shows a lobulating mass forming colocolic intussusception (arrows). The mass shows a heterogeneous less enhancement feature and thin-enhancing inner layer suggesting that the mucosa is well-preserved (arrowheads). There is no lymph node enlargement or pericolic fat infiltration.
E. Axial 18F-FDG PET/CT image shows an increased FDG-uptake (SUV max = 5.8) around the mid ascending colon (arrow) without evidence of metastasis.
F. Gross pathological cut section shows a whitish tumor without necrosis or hemorrhage.
G. The microscopic exam (hematoxylin and eosin stain, × 100) of the mass shows dense bundles of short spindle cells (arrows) and collagen fibers.
H. In immunohistochemistry, the stain is positive for beta catenin (× 100) and negative for C-kit (× 100) and CD34 (× 100), which is very helpful in distinguishing mesenteric fibromatosis from other similar lesions.
FDG = fluorodeoxyglucose, PET = positron emission tomography, SUV max = maximum standardized uptake value
References
1. Kasper B, Ströbel P, Hohenberger P. Desmoid tumors: clinical features and treatment options for advanced disease. Oncologist. 2011; 16:682–693.
2. Levy AD, Rimola J, Mehrotra AK, Sobin LH. From the archives of the AFIP: benign fibrous tumors and tumorlike lesions of the mesentery: radiologic-pathologic correlation. Radiographics. 2006; 26:245–264.
3. Sheth S, Horton KM, Garland MR, Fishman EK. Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. Radiographics. 2003; 23:457–473. quiz 535-536.
4. Shinagare AB, Ramaiya NH, Jagannathan JP, Krajewski KM, Giardino AA, Butrynski JE, et al. A to Z of desmoid tumors. AJR Am J Roentgenol. 2011; 197:W1008–W1014.
5. Ghai S, Pattison J, Ghai S, O'Malley ME, Khalili K, Stephens M. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics. 2007; 27:1371–1388.
6. Horton KM, Abrams RA, Fishman EK. Spiral CT of colon cancer: imaging features and role in management. Radiographics. 2000; 20:419–430.
7. Metser U, Even-Sapir E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): accumulated data from four years of experience with PET/CT. Semin Nucl Med. 2007; 37:206–222.
8. Lo KW. Mesenteric fibromatosis as a potential source of false-positive interpretation of FDG-PET: report of a case. Dis Colon Rectum. 2007; 50:924–926.
9. Montgomery E, Torbenson MS, Kaushal M, Fisher C, Abraham SC. Beta-catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol. 2002; 26:1296–1301.
10. Choi SH, Han JK, Kim SH, Lee JM, Lee KH, Kim YJ, et al. Intussusception in adults: from stomach to rectum. AJR Am J Roentgenol. 2004; 183:691–698.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download