Abstract
Purpose
To determine the efficacy of embolization with super-selective catheterization of the internal iliac arterial branches to treat heavily injured trauma patients with pelvic arterial bleeding.
Materials and Methods
A retrospective analysis was performed using the medical records of 37 patients who underwent trans-arterial embolization for trauma-associated pelvic arterial hemorrhage in a regional trauma center between July 2010 and July 2013. In each patient, hemodynamic stability, embolization level, elapsed time for trans-arterial embolization, clinical outcome and embolization-related complications were evaluated. Comparison of elapsed time, and presence of complication was done according to embolization level.
Results
Transarterial embolization was conducted in 37 patients. Hemodynamic stability of each patient was classified into hemodynamic stable (n = 9), and unstable (n = 28). Twenty-nine of 37 patients underwent embolization after super-selective catheterization of more than 2nd order branch of internal iliac artery with a microcatheter, and 8 patients underwent proximal internal iliac artery embolization without super-selective catheterization. The mean elapsed procedure time for super-selective embolization (34.76 ± 20.0 minutes) was not significantly longer than proximal internal iliac artery embolization (33.87 ± 16.73 minutes, p = 0.215).
Transcatheter arterial embolization (TAE) has become an accepted treatment modality for trauma-associated active arterial bleeding in various organs, because it can rapidly treat not only single arterial bleeding but also multiple and anatomically distant bleeding sites from a single arterial access (1). Currently, despite ongoing debates, TAE for bleeding pelvic trauma in hemodynamically unstable patients is regarded as a fundamental treatment option with open surgery, like pelvic packing (1234). Notably, according to the Eastern Association for Surgery's pelvic trauma guideline, emergent pelvic angiography and embolization in patients with unstable hemodynamic status has the highest level of recommendation (5).
Although TAE is one of the most useful modalities for controlling life-threatening hemorrhage after complex pelvic trauma, it may be associated with various complications, including puncture site hematoma, impotence, iliofemoral embolization, paraparesis, and ischemic necrosis of the bladder wall, gluteal skin, femoral head, gluteal muscle, and colon (678910111213). Super-selective catheterization of injured vessels may be a good option for avoiding complications related to TAE, because the blood flow to adjacent structures can be maintained by selectively embolizing only the injured vessel. However, super-selective catheterization of injured vessel is regarded as a time-consuming step and may therefore be unreasonable to perform in patients with unstable hemodynamics due to massive bleeding (6). In this retrospective study, we aimed to determine whether re-evaluation of TAE after super-selective catheterization is necessary for treatment of life-threatening pelvic hemorrhage after trauma. In addition, we evaluated the possibility of super-selective embolization in patients showing unstable hemodynamics after severe pelvic trauma.
A total of 37 patients (mean age, 59.8 years; male:female ratio, 12:25) visited our trauma center with arterial hemorrhage after pelvic bone fracture and underwent angiography and embolization between July 2010 and July 2013. For each patient who underwent angiography and embolization, medical records were reviewed to assess the following clinical and hemodynamic parameters: age; sex; elapsed time for embolization; hemoglobin concentration at the time of the first presentation and just before embolization; systolic blood pressure at the time of the first presentation and before embolization; amount of blood transfused (total amount of packed red blood cells); and the amount of fluids administered intravenously.
Hemodynamic stability for each patient was assessed according to a modified hemodynamic instability score as proposed in 2008 by the Western Trauma Association (14). Systolic blood pressure < 90 mm Hg defined as significant hypotension and a heart rate > 130 beat per minute defined as serious tachycardia was based on the recently published guidelines for shock resuscitation developed by the National Institutes of Health-sponsored Glue Grant consortium (15). Based on this scoring system, patients were categorized as stable patients (grades 0, 1, and 2) or unstable patients (grades 3, 4, and 5). Comparison of measured injury severity score (ISS) was done in all patients for evaluation of severity. The ISS is an anatomical scoring system that provides an overall score for patients with multiple injuries. Each injury is assigned an Abbreviated Injury Scale (AIS) and is allocated to 1 of 6 body regions (head, face, chest, abdominal or pelvic contents, extremities or pelvic girdle, external). AIS is an anatomical scoring system in which injuries are ranked on a scale of 1 to 6, with 1 as minor, 5 critical, and 6 a currently untreatable injury. The AIS score of 3 most severely injured body regions is squared and summed to produce the ISS score. The formula for ISS calculation is ISS = (AIS in B1)2 + (AIS in B2)2 + (AIS in B3)2, where BN stands for body regions.
Angiography and embolization was performed whenever active bleeding was suspected based on pelvic computed tomographic angiography (CTA) or in cases in which ongoing arterial bleeding was strongly suspected, regardless of CTA findings. After local anesthesia with 2% lidocaine, all patients underwent transfemoral arterial catheterization. After catheterization, digital subtraction pelvic angiography was performed using a 5-Fr pig-tail catheter (Merit Medical, South Jordan, UT, USA) to check for the presence of lesions, as well as to assess the anatomy of blood vessels. Catheterization of the internal iliac artery was attempted with a 5-Fr cobra-type catheter (Cook, Bloomington, IN, USA) or 5-Fr Yashiro-type catheter (Terumo, Tokyo, Japan). To decrease the time required for selecting each internal iliac artery, overlay image guides were used if the initial attempt to select the internal iliac artery failed. In all patients, angiography was done using the digital angiography system, Allura Xper FD20 (Philips Medical Systems, Best, the Netherlands). Overlay image was obtained from road-map or smart mask (Philips Medical Systems). For smart mask, correctly obtained angiographic image was selected for projection overlay on the fluoroscopic screen. The embolization level was decided by the interventional radiologist who performed the angiography. After pelvic arteriogram, anatomical accessibility toward injured vessel, patient's possibility of cooperation and degree of additional traumatic injury were considered comprehensively. Subsequently, super-selective embolization was attempted when possible; if not, proximal embolization was conducted.
Arterial catheterization and embolization level was recorded for each patient. Super-selective embolization was defined as embolization after catheterization of an arterial branch that was more than 2nd order level. A microcatheter (Renegade STC 18, Boston Scientific, Natick, MA, USA) was used for super-selective catheterization. Proximal embolization was defined as embolization at proximal internal iliac artery and 1st order branch from internal iliac artery. Active bleeding or traumatic vascular injury on angiography was defined as > 1 of the following angiography findings: 1) extravasation or blushing of contrast agent, 2) abrupt cut off of arterial branch, or 3) dissection of blood vessel.
An absorbable gelatin sponge (Cutanplast; Mascia Brunelli S.p.A, Milan, Italy) or microcoils (Tornado coil; Cook, Bloomington, IN, USA) were used together or separately for post-catheterization hemostasis. In patients needing proximal embolization, only an absorbable gelatin sponge was used as a gelfoam slurry. A 5-Fr angiocatheter was used in all cases with proximal embolization. Complete exclusion of arterial bleeding on final angiogram was defined as technical success.
The feasibility and safety of embolization after super-selective catheterization was based on the following: elapsed time between first angiography after femoral artery catheterization and final angiography after embolization in each patient and the development of complications associated with pelvic arterial embolization (pelvic ischemia or paresis). Elapsed time was calculated from automatically recorded time by a picture archiving communication system. Comparison of elapsed procedure times according to embolization levels was by t-test and logistic regression test was used to check whether the difference between elapsed procedure times influences the patient outcome. All statistical analyses were done with the software statistical package for social science version 21.0 (SPSS Inc., Chicago, IL, USA). This study was approved by our Institutional Review Board.
The medical records of all patients who underwent TAE for trauma associated pelvic hemorrhage, were summarized in Table 1.
According to the modified hemodynamic instability score as proposed by the Western Trauma Association in 2008, 9 patients were classified as hemodynamically stable and 28 were classified as hemodynamically unstable (Table 2). The mean ISS was 25.29 ± 8.74. The mean ISSs of these 2 groups were 25.33 ± 9.03 vs. 25.29 ± 8.82 (hemodynamically stable group vs. hemodynamically unstable group, respectively), without significant differences (p = 0.989).
Thirty-three of 37 patients who underwent pre-embolization CTA, showed obvious extravasation. Despite the absence of extravasation at CTA, angiography and subsequent embolization was performed in 4 patients for the following reasons: extensive hematoma and hemoretroperitoneum around pelvic bone fracture (n = 2), continued decrease in hemoglobin level even after transfusion in pelvic bone fracture patient (n = 1), and priapism after trauma (n = 1).
Forty-eight arterial branches were embolized in the 37 enrolled patients. In 8 patients, TAE was performed in > 2 arterial branches. Among the 37 patients, 29 were embolized after super-selective catheterization of a more than 2nd order branch of the internal iliac artery (Fig. 1). The remaining 8 patients were embolized at the proximal internal iliac artery (n = 7) and at a 1st order branch of the internal iliac artery (n = 1) (Fig. 2). Immediate hemostasis was achieved in all patients regardless of the level of embolization, and the technical success rate was 100% for transarterial embolization for the treatment of trauma-associated pelvic arterial bleeding. Embolized arteries were listed in Table 3.
Elapsed procedure time in each patient was shown in Table 1. The mean elapsed procedure time between the first angiography after femoral catheterization and the final angiography after embolization for all subjects was 34.57 ± 19.12 minutes. The mean elapsed procedure time for TAE with super-selective catheterization (34.76 ± 20.0 minutes) was slightly longer than the elapsed time for proximal embolization (33.87 ± 16.73 minutes). However, there was no statistically significant difference in procedure time (p = 0.215). For patients with unstable hemodynamics, the mean elapsed procedure time for TAE was not significantly different between the super-selective catheterization (n = 21, 35.43 ± 20.1 minutes) and proximal embolization groups (n = 7, 35.86 ± 13.36 minutes) (p = 0.770). Elapsed procedure time according to hemodynamic stability were summarized in Table 4.
In addition, binary logistic regression test showed that the elapsed procedure time for super-selective catheterization did not influence the mortality rate for patients with unstable hemodynamics (p = 0.077; odds ratio, 1.046).
The in-hospital mortality rate was 24.3%; i.e., 9 patients died after TAE; and all mortality cases were hemodynamically unstable. Causes of death were as follows: combined brain injury (n = 4), combined multiorgan injury (n = 2), disseminated intravascular coagulopathy after bleeding and transfusion (n = 1), hypovolemic shock (n = 1), and pulmonary thromboembolism after sustained bed rest after 1 month (n = 1). Causes of in-hospital mortality according to level of embolization were listed in Table 5. A complication that was possibly related to embolization of the pelvic cavity developed in 1 patient who underwent embolization without super-selective catheterization. This patient produced a weak urine stream after TAE of the bilateral proximal internal iliac artery.
Definitive control of arterial bleeding associated with pelvic fracture in the heavily injured patient can be difficult even for experienced physicians. Although various methods for controlling life-threatening pelvic bleeding, such as pneumatic anti-shock garments, external skeletal fixation, immediate open reduction and internal fixation, pelvic packing, and pelvic external fixation, have all been used for controlling blood loss, retroperitoneal hemorrhage resulting from pelvic fracture is difficult to control, and mortality remains high (239).
TAE is now accepted as a good treatment method for trauma patients with pelvic bleeding (1234561617). Post-pelvic angiography embolization may be possible after with or without super-selective catheterization of the injured branch. Super-selective embolization is a well-established method for preventing unexpected complications after blind embolization of pelvic circulation in patients with stable hemodynamics. On the other hand, controversy remains over the feasibility of super-selective embolization in patients showing unstable hemodynamics (245). The interventional radiologist is required to balance the need for rapid treatment and embolization of the actual bleeding vessel super-selectively, which could be a time-consuming task.
Several reports have indicated the complications related to proximal embolization, without super-selective catheterization, of the internal iliac artery for trauma patients (6). Gluteal muscle necrosis after proximal embolization at the internal iliac artery level is most frequently reported, and its estimated incidence is about 6% (7810111213). Surgical wound break down (12), pelvic organ infarction, including colon, ileum, ureter, and rectum (79), and neurologic complications, such as lower limb paresis, sacral plexus palsy, and sciatic palsy (9), have also been reported. Such complications could require additional surgical procedures, longer hospitalization, and lead to unexpected results, thus negatively impacting the trauma patient's outcome. In our series, 1 patient who underwent bilateral proximal internal iliac artery embolization experienced weak urine stream after treatment. There were no complications related to embolization after super-selective catheterization.
Currently, multi-detector CTA facilitates rapid and exact image diagnosis for trauma patients (181920). Recently developed technically advanced angiography machines equipped with high-resolution digital flat panel detectors are now widely used. As a result, initial evaluation for trauma patients more accurately detects the injured vessel, even in hemodynamically unstable patients. Particularly, overlay imaging techniques with high quality images make it possible to have more rapid guide-wire negotiation and catheterization toward the injured vessel in the complex pelvic vasculature. In our series, the injured vessel was catheterized super-selectively, under overlay imaging guidance in 29 patients. Although the procedure time for super-selective catheterization can be slightly longer than for proximal embolization, the elapsed time for the procedures were not significantly different.
There were 2 interesting results from our analysis of 28 patients who presented with unstable hemodynamics. First, there were no significant differences in elapsed procedure time, between the patient undergoing super-selective embolization distal to a 2nd order internal iliac artery branch and the patients undergoing proximal embolization. Second, binary logistic regression analysis for the mortality of patients with unstable hemodynamics revealed that there was no significant correlation with elapsed procedure time. These findings suggest that super-selective TAE for pelvic hemorrhage is not a time consuming procedure and can be done as rapidly as proximal embolization, without lowering the survival rate for trauma victims. In our experience, proximal embolization requires the use of more embolic agents, such as gelfoam slurry, and more time to occlude the vessel, as compared to embolization after super-selective catheterization of the injured vessel.
Our retrospective study had some limitations for comparing the 2 procedures, including the small number of patients and the inability to randomize treatments due to the characteristics of the trauma patients.
In conclusion, TAE after super-selective catheterization for patients with pelvic hemorrhage after multifocal pelvic trauma did not significantly increase procedure time, even in patients with unstable hemodynamics. Super-selective TAE for unstable patients showed no negative effect on mortality or ischemic complications related to proximal internal iliac artery embolization. Thus, super-selective TAE for pelvic arterial hemorrhage is a feasible and safe method for controlling bleeding for both hemodynamically stable and unstable patients.
Figures and Tables
Fig. 1
Embolization after super-selective catheterization. A 61-year-old woman (case no. 20) treated with embolization of the right internal pudendal artery presented with unstable hemodynamics (hemodynamic instability score = 4).
A. Axial contrast-enhanced computed tomography shows extravasation around the right side of the pelvic wall (arrow).
B, C. Angiography shows active bleeding from the small branch of the internal pudendal artery. The microcatheter is introduced into the internal pudendal artery (arrowheads).
D. Angiogram after coil embolization shows complete exclusion of bleeding.
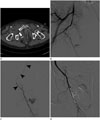
Fig. 2
Embolization without super-selective catheterization. A 73-year-old woman (case no. 24) treated with embolization of the bilateral proximal internal iliac artery using gelfoam slurry presented with unstable hemodynamics (hemodynamic instability score = 4).
A. Initial pelvic angiography shows multifocal hemorrhages from the bilateral internal iliac artery branches (arrows).
B. Angiogram obtained after embolization of the proximal internal iliac artery shows complete exclusion of bleeding site. This patient did not survive after embolization due to complications from a combined hypoxic brain injury.
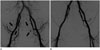
Table 1
Patient Characteristics

Table 2
Hemodynamic Stability

Table 3
Embolized Artery

Table 4
Elapsed Procedure Time

Table 5
Clinical Outcome after Embolization

References
1. Bize PE, Duran R, Madoff DC, Golliet-Mercier N, Heim C, Pilleul F, et al. Embolization for multicompartmental bleeding in patients in hemodynamically unstable condition: prognostic factors and outcome. J Vasc Interv Radiol. 2012; 23:751–760.e4.
2. Suzuki T, Smith WR, Moore EE. Pelvic packing or angiography: competitive or complementary? Injury. 2009; 40:343–353.
3. Marzi I, Lustenberger T. Management of Bleeding Pelvic Fractures. Scand J Surg. 2014; 103:104–111.
4. Fu CY, Wang YC, Wu SC, Chen RJ, Hsieh CH, Huang HC, et al. Angioembolization provides benefits in patients with concomitant unstable pelvic fracture and unstable hemodynamics. Am J Emerg Med. 2012; 30:207–213.
5. Cullinane DC, Schiller HJ, Zielinski MD, Bilaniuk JW, Collier BR, Como J, et al. Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture--update and systematic review. J Trauma. 2011; 71:1850–1868.
6. Frevert S, Dahl B, Lönn L. Update on the roles of angiography and embolisation in pelvic fracture. Injury. 2008; 39:1290–1294.
7. Suzuki T, Kataoka Y, Minehara H, Nakamura K, Uchino M, Kawai K, et al. Transcatheter arterial embolization for pelvic fractures may potentially cause a triad of sequela: gluteal necrosis, rectal necrosis, and lower limb paresis. J Trauma. 2008; 65:1547–1550.
8. Takahira N, Shindo M, Tanaka K, Nishimaki H, Ohwada T, Itoman M. Gluteal muscle necrosis following transcatheter angiographic embolisation for retroperitoneal haemorrhage associated with pelvic fracture. Injury. 2001; 32:27–32.
9. Perez JV, Hughes TM, Bowers K. Angiographic embolisation in pelvic fracture. Injury. 1998; 29:187–191.
10. Suzuki T, Shindo M, Kataoka Y, Kobayashi I, Nishimaki H, Yamamoto S, et al. Clinical characteristics of pelvic fracture patients with gluteal necrosis resulting from transcatheter arterial embolization. Arch Orthop Trauma Surg. 2005; 125:448–452.
11. Gilleard O, Stammers J, Ali F. Gluteal necrosis following pelvic fracture and bilateral internal iliac embolization: reconstruction using a transposition flap based on the lumbar artery perforators. Int J Surg Case Rep. 2012; 3:86–88.
12. Matityahu A, Marmor M, Elson JK, Lieber C, Rogalski G, Lin C, et al. Acute complications of patients with pelvic fractures after pelvic angiographic embolization. Clin Orthop Relat Res. 2013; 471:2906–2911.
13. Yasumura K, Ikegami K, Kamohara T, Nohara Y. High incidence of ischemic necrosis of the gluteal muscle after transcatheter angiographic embolization for severe pelvic fracture. J Trauma. 2005; 58:985–990.
14. Moore FA, Davis JW, Moore EE Jr, Cocanour CS, West MA, McIntyre RC Jr. Western Trauma Association (WTA) critical decisions in trauma: management of adult blunt splenic trauma. J Trauma. 2008; 65:1007–1011.
15. Moore FA, McKinley BA, Moore EE, Nathens AB, West M, Shapiro MB, et al. Inflammation and the Host Response to Injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care. III. Guidelines for shock resuscitation. J Trauma. 2006; 61:82–89.
16. Tanizaki S, Maeda S, Hayashi H, Matano H, Ishida H, Yoshikawa J, et al. Early embolization without external fixation in pelvic trauma. Am J Emerg Med. 2012; 30:342–346.
17. Velmahos GC, Toutouzas KG, Vassiliu P, Sarkisyan G, Chan LS, Hanks SH, et al. A prospective study on the safety and efficacy of angiographic embolization for pelvic and visceral injuries. J Trauma. 2002; 53:303–308. discussion 308
18. Pinto A, Niola R, Tortora G, Ponticiello G, Russo G, Di Nuzzo L, et al. Role of multidetector-row CT in assessing the source of arterial haemorrhage in patients with pelvic vascular trauma. Comparison with angiography. Radiol Med. 2010; 115:648–667.
19. Nicholson AA. Vascular radiology in trauma. Cardiovasc Intervent Radiol. 2004; 27:105–120.
20. Salazar GM, Walker TG. Evaluation and management of acute vascular trauma. Tech Vasc Interv Radiol. 2009; 12:102–116.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download