Abstract
Epipericardial fat necrosis (EFN) is an infrequent cause of acute chest pain. In rare cases like these, conservative treatment is necessary. Clinically, EFN may mimic emergent cardiopulmonary conditions, such as acute myocardial infarction. Computed tomography (CT) and magnetic resonance imaging characteristics of EFN is well described as encapsulated fatty lesion with perilesional soft tissue strands and thickening of adjacent pericardium in the epipericardial area. For confirmation of the diagnosis, involution of this lesion on follow-up is important. We present a case of EFN observed with ultrasonography (USG). This lesion was shown as a well-defined ovoid shaped mass with heterogeneous echogenicity in the left side of cardiophrenic space on USG. There was no color flow on Doppler USG. Follow-up USG and CT revealed decrease in the size of the lesion.
Epipericardial fat necrosis (EFN) is an uncommon benign and self-limited disease, which was initially described by Jackson et al. (1). It presents with acute chest pain and can mimic serious diseases, such as acute myocardial infarction or pulmonary thromboembolism (2). Computed tomography (CT) characteristics of EFN are well described as the encapsulated fatty lesion with perilesional strands and thickening of adjacent pericardium in the epipericardial area (3). Magnetic resonance imaging (MRI) also might be helpful for distinguishing EFN from other causes of acute chest pain or fat-containing tumors (4). For confirming the diagnosis, the involution of this lesion on follow-up is important. Serial CT or MRI is usually used for follow-up in the previous reports (34).
To the best our knowledge, this is the first reported case of EFN evaluated with ultrasonography (USG). Here, we describe initial presentation and follow-up USG findings of EFN, and correlated these with CT findings.
A 31-year-old man was admitted to our hospital due to sudden onset of left-sided pleuritic chest pain. He did not have other symptoms, such as fever or dyspnea. His physical exam and laboratory tests were all within normal ranges.
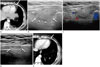
The chest radiograph was unremarkable. Chest CT demonstrated an encapsulated fat attenuated lesion with surrounding enhancing strands in the left cardiophrenic space. Thickening and enhancement of the adjacent pericardium and pleura was also identified. A small pleural effusion in the left hemithorax was noted (Fig. 1A). On the USG performed through the intercostal spaces with a low frequency convex probe, an ovoid mass with slightly lower echogenicity compared to the adjacent mediastinal fat was visualized in the left cardiophrenic space. With a high frequency linear probe, this lesion was shown as a well-defined ovoid mass with heterogeneous internal echogenicity, a slightly hyperechoic rim, and surrounding low echoic halo (Fig. 1B). There was no color flow within the mass on Doppler USG (Fig. 1C).
After consideration of the clinical and radiologic findings, provisional diagnosis of EFN was made and his symptoms subsided with conservative treatment. Two months later, follow-up USG revealed a decrease in the size of the lesion and the perilesional low echoic halo was resolved (Fig. 1D). Chest CT also showed a decreased in the lesion size and improvement of perilesional fat infiltration and pericardial thickening (Fig. 1E).
Fat necrosis can occur in various sites of the body. EFN has similar clinical and pathologic features with fat necrosis seen elsewhere in the body. Conservative treatment is indicated due to EFN's benign self-limiting nature, like other fat necroses (3). Due to the characteristic epipericardial location of fat necrosis, most of the patients with EFN present with pleuritic chest pain of sudden onset, which can mimic an acute cardiopulmonary process.
The pathogenesis of EFN is unclear. Jackson et al. (1) initially proposed that obesity could be a predisposing factor, but recent reports have shown that it can also occur in non-obese individuals (25). Our patient had body mass index value in the normal range. Three theories for pathogenesis of EFN are proposed in the literature. Ischemic necrosis of the epipericardial fat caused by acute torsion is proposed and the presence of a vascular pedicle has been described in two cases (6). The second theory is that elevated thoracic pressure, caused by a Valsalva's maneuver, may increase capillary pressure and may result in the consequent hemorrhagic necrosis within the epipericardial fatty area (3). It also has been hypothesized that the presence of a structural abnormality, such as a lipoma or hamartoma, increases susceptibility to heartbeat and diaphragmatic movement trauma (7).
The diagnosis of EFN is confirmed by surgical resection and pathologic examination in case reports prior to 2004. Increased utilization of cross-sectional imaging, such as CT and MRI, characteristic radiologic findings and the spontaneous involution of EFN have been well described (348). In most recent EFN cases, together with our patient's clinical presentation, radiologic examination and follow-up imaging may be sufficient to confirm the diagnosis, and conservative management is considered the treatment of choice (38). Therefore, it is important for the radiologists to know the radiologic characteristics of EFN for appropriate diagnosis, to avoid unnecessary surgery.
Radiologic findings of EFN have been described as case reports. Although a juxta-cardiac mass with or without pleural effusion may be seen, chest radiography can be normal (7). Most common CT findings are encapsulated fatty lesions with internal dense strands. Perilesional mediastinal fat stranding and thickening of the adjacent pericardium may be observed as well (37). EFN may be identified by MRI as a fat containing soft tissue lesion with a peripheral rim and low signal central dot-and-line on the T1- and T2-weighted images. Rim enhancement may be noted on gadolinium enhanced T1-weighted image, as well (4). Our patient exhibited similar CT and MRI findings in the characteristic locations for EFN.
To the best of our knowledge, USG findings of EFN has not been reported. USG is rarely performed for EFN diagnosis, because the sonic window is expected to be poor due to the bony thoracic cage and adjacent pulmonary air. However, considering the typical location of EFN, which is in the epipericardial fat pad posterior to the anterior chest wall, this lesion may be evaluated with USG if there is no intervening lung. On the USG of our patient, this lesion is shown as a well-defined ovoid mass with heterogeneous internal echogenicity, slightly hyperechoic rim and perilesional low echoic halo. A spontaneous decrease in size of this lesion was also well documented by follow-up USG and CT in our patient. USG has several advantages over CT and MRI, including the easy accessibility and absence of radiation exposure. USG may be used as a diagnostic or follow-up imaging method for EFN.
EFN shares many ultrasonographic features with fat necrosis seen elsewhere in the body, such as epiploic appendagitis. In a previous study, patient abdominal USG images of epiploic appendagitis have shown an oval, hyperechoic, non-compressible mass surrounded by a low echoic halo in the left lower abdomen and at the point of maximum tenderness (9). In our patient, similar findings were shown on the USG at the left lower anterior chest wall. Moreover, neither of them were demonstrated internal flow on Doppler USG, which may suggest pathogenesis secondary to ischemia (9). In addition, this finding may be helpful for distinguishing EFN from fat containing tumors. Heterogeneous internal echogenicity of EFN on USG is likely due to internal fat component and hemorrhagic necrosis, like epiploic appendagitis (10).
In conclusion, we have described the ultrasonographic and CT findings in a case of EFN. It is important for radiologists to be aware of these radiologic findings in EFN to avoid unnecessary invasive procedures. By demonstrating a fatty mass that decreased in size on follow-up, USG can be used as a diagnostic or follow-up imaging method for EFN with the better accessibility and absence of radiation exposure.
Figures and Tables
Fig. 1
A 31-year-old male patient with sudden left pleuritic chest pain.
A. Enhanced chest CT scan shows that the encapsulated ovoid lesion with internal fat attenuation (arrow), surrounding strands and pericardial thickening (arrowheads) in the left cardiophrenic space. A small pleural effusion is noted in left hemithorax (asterisk).
B. USG image through the intercostal space shows an ovoid mass (arrows) with internal heterogeneous echogenicity and surrounding low echoic halo (arrowheads) in the left side of the epipericardial area.
C. There is no color flow within the mass on the Doppler USG.
D. Follow-up USG obtained two months later demonstrates an interval decrease in lesion size (arrows) with a variable change of the internal echogenicity, as well as resolution of perilesional low echoic halo.
E. Follow-up enhanced chest CT scan obtained two months later shows an interval decrease in lesion size (arrow) and resolution of the surrounding strands and pleural effusion in left hemithorax.
CT = computed tomography, USG = ultrasonography
References
1. Jackson RC, Clagett OT, McDonald JR. Pericardial fat necrosis; report of three cases. J Thorac Surg. 1957; 33:723–729.
2. Behrendt DM, Scannell JG. Pericardial fat necrosis. An unusual cause of severe chest pain and thoracic "tumor". N Engl J Med. 1968; 279:473–475.
3. Pineda V, Cáceres J, Andreu J, Vilar J, Domingo ML. Epipericardial fat necrosis: radiologic diagnosis and follow-up. AJR Am J Roentgenol. 2005; 185:1234–1236.
4. Lee HH, Ryu DS, Jung SS, Jung SM, Choi SJ, Shin DH. MRI findings of pericardial fat necrosis: case report. Korean J Radiol. 2011; 12:390–394.
5. Bensard DD, St Cyr JA, Johnston MR. Acute pleuritic chest pain and lung mass in an elderly woman. Chest. 1990; 97:1473–1474.
6. Webster MW Jr, Bahnson HT. Pericardial fat necrosis. Case report and review. J Thorac Cardiovasc Surg. 1974; 67:430–443.
7. Ataya D, Chowdhry AA, Mohammed TL. Epipericardial fat pad necrosis: computed tomography findings and literature review. J Thorac Imaging. 2011; 26:W140–W142.
8. Pineda V, Andreu J, Cáceres J, Merino X, Varona D, Domínguez-Oronoz R. Lesions of the cardiophrenic space: findings at cross-sectional imaging. Radiographics. 2007; 27:19–32.
9. Mollá E, Ripollés T, Martínez MJ, Morote V, Roselló-Sastre E. Primary epiploic appendagitis: US and CT findings. Eur Radiol. 1998; 8:435–438.
10. Rioux M, Langis P. Primary epiploic appendagitis: clinical, US, and CT findings in 14 cases. Radiology. 1994; 191:523–526.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download