Abstract
Purpose
To assess the imaging and clinicopathologic outcomes of recurrent and second breast cancer after breast-conserving surgery for invasive ductal carcinomas detected on follow-up mammography and breast ultrasound (US).
Materials and Methods
Seventy-six women with an ipsilateral breast tumor recurrence (IBTR) or regional lymph node recurrence and/or contralateral breast cancer (RLNR & CBC) after breast-conserving surgery were included in this study. The mammography and US images were analyzed and the clinicopathologic parameters were compared between the groups.
Results
Thirty had an IBTR, and 46 had a RLNR & CBC. The IBTR group's mammography and US images frequently revealed calcification and masses on the breast, respectively. The most frequent site of RLNR detected during follow-up mammography and breast US was the axilla. In univariate analysis, the tumors in the IBTR group were predominantly estrogen receptor (ER)-negative, HER-2 overexpression, and p53-positive. ER and HER-2 were shown by the multivariate analysis to be independent parameters associated for both types of recurrences.
The breast-conserving operation is the established treatment modality for early breast cancer, with a long-term survival equal to radical surgery (12). Early detection of an ipsilateral breast tumor recurrence (IBTR) and recurrence in regional lymph nodes has a beneficial impact on local disease control and survival of breast cancer patients when compared to late symptomatic detection (3).
The risk factors associated with IBTR and regional recurrence include younger patient age (456), larger tumor size, multifocal multi-centric disease (7), close or positive margins (68), an extensive intraductal component (49), higher tumor grade (10), the presence of lymphovascular invasion (9), negative histology for estrogen receptor (ER) or progesterone receptor (PR) expression, and omission of radiation (1112). The current recommendations for postoperative follow-up for breast-conserving surgery following an invasive ductal carcinoma are to take the patient's history, a clinical breast examination, and an annual mammogram of the breast tissues (13). Unlike recurrences after mastectomy, which are typically diagnosed by physical examination, IBTRs after a breast-conserving operation are detected by mammography alone (14). In addition, breast ultrasound (US) is the best imaging method for evaluating a regional recurrence, which cannot be visualized by mammography (151617).
The purpose of our study is to correlate the post-surgical follow-up mammography and US imaging results with clinicopathologic factors of recurrent and second breast cancer–IBTR, or regional lymph node recurrence and/or contralateral breast cancer (RLNR & CBC) after breast-conserving surgery for invasive ductal carcinoma.
This retrospective study was approved by our hospital's Institutional Review Board for human investigation, and informed consent was waived for use of the data.
A hospital electronic medical record query identified 5521 women that underwent surgery for breast cancer from January 2009 to December 2011. Among these cases, we consecutively enrolled patients who: 1) had undergone breast-conserving surgery for invasive ductal carcinoma, 2) had routine follow-up mammography or US every six months or one year after surgery, and 3) had an IBTR or an RLNR & CBC detected by mammography or breast US. The recurrence pathology was confirmed by an US-guided core needle biopsy or fine needle aspiration biopsy, stereotactic biopsy, or surgery. Finally, 76 women (24–75 years of age, mean age ± standard deviation: 46.9 ± 10.1 years) were included in our analyses.
Mammography performed for diagnosis was obtained using the Senographe DS unit (GE Healthcare, Milwaukee, WI, USA). All women underwent a two-view mammography (i.e., craniocaudal and mediolateral oblique views). Whole-breast US was performed by board-certified radiologists with variable clinical experience of breast US ranging from 2 to 20 years, using an IU22 (Philips Healthcare, Bothell, WA, USA) equipped with a 50-mm, linear array transducer with a bandwidth of 7–12 MHz. At our institution, the scanning technique for bilateral whole-breast US was standardized as follows: the procedure begins with scanning of the right breast using transverse and sagittal orientations, scanning of the inner breast occurs in a supine position, and the outer breast is performed in a supine oblique position with the woman's arm raised above her head. In addition, both axilla, supraclavicular fossa, and internal mammary lymphatic space were scanned for the detection of regional or metastatic lymph nodes. This procedure is then repeated on the left breast.
Two radiologists (J.H.C. and W.J.C.) with 16 and 7 years of breast cancer clinical expertise, respectively, reviewed the mammography and US results at the time of the recurrence in consensus. The mammographic results were categorized as follows: mass, mass with calcification, calcification alone, focal asymmetry, lymph node enlargement in the axilla, and post-operative results only. The US findings were classified as mass; mass with calcification; lymph node enlargement in the axilla, internal mammary lymphatic space, or supraclavicular area; and no evidence of recurrence.
The histopathological reports of the initial breast cancers were reviewed, and the following characteristics were recorded: histological subtype, tumor size, nodal status, nucleus and histologic grades, American Joint Committee on Cancer stage, ER and PR receptor expression, HER-2 expression, and immunohistochemical subtype.
Univariate and multivariate statistical analyses were performed to determine whether there was a correlation between the IBTR and RLNR & CBC groups and the clinicohistopathological factors, as well as to determine the independent risk factors for recurrence. The associations of the variables were assessed using the chi-square test. Variables with probability values less than 0.1 were included in the multivariate analysis, using a logistic regression analysis with backward elimination. A p value less than 0.1 was considered to be statistically significant. All statistical analyses were performed with SPSS statistical software for Windows, version 20.0 (IBM Corp., Armonk, NY, USA).
All 76 women involved in this study had undergone a breast-conserving operation for invasive ductal carcinoma. At the initial operation, the median lesion size of the tumor was 22 mm (range, 5–52 mm). Twenty-three patients had a positive nodal status, and seven patients had tumors with a positive resection margin. The overall median interval from the operation to recurrence was 23 months (range, 2–60 months). The interval for an IBTR was 21 months (2–60 months), and that for a RLNR & CBC was 24 months (7–57 months). Thirty patients developed an IBTR, and 46 patients had a RLNR & CBC. In the RLNR & CBC group, 15 patients had breast cancer in the contralateral breast, and the remaining 31 patients had a regional lymph node metastasis.
For the breast evaluation, 22 mammography and 29 US procedures were performed in the IBTR group. Tewnty-nine mammography and 45 US examinations were performed in the RLNR & CBC group. Six patients had tumors detected in the mammography only group, 48 in the US only group, and 22 in the group with both types of breast imaging. Table 1 summarizes the lesion types via mammography and US between the IBTR and RLNR & CBC groups. The most frequent imaging findings were calcification with or without a mass by mammography for IBTR (n = 13, 17.1%) and a mass with or without calcification by US (n = 25, 32.9%) (Fig. 1).
Of the 30 patients in the IBTR group, including all three patients with a previous positive resection margin at initial operation, 27 patients had a recurrence near the previous operation site. The interval period of three patients with a previous positive resection margin was 12 months, 34 months, and 60 months, respectively. The recurred lesions were newly developed calcifications or mass with calcifications. The most frequent site of regional lymph node metastasis was the axilla, followed by the supraclavicular lymph node and internal mammary lymph node (Fig. 2).
Both the IBTR and RLNR & CBC groups were more frequently associated with younger age, smaller tumor size, negative node status, higher nuclear and histologic grades, triple negative (ER-, PR-, and HER-2-) status, and the basal-like subtype. In univariate analysis for differentiation of the IBTR and RLNR & CBC groups, the IBTR group showed a significantly higher fraction of ER-negative, HER-2 overexpressing, and p53-positive tumors (Table 2). There was no significant association between IBTR and RLNR & CBC groups in terms of patient age, initial tumor size, nodal status, nuclear grade, histologic grade, or PR status. From multivariate analysis, ER and HER-2 were identified as independent parameters associated for both types of cancer recurrences.
Breast-conserving operations have become the established treatment modality for early breast cancer (12). Early detection of IBTRs and RLNR & CBCs has a beneficial impact on local disease control and breast cancer patient survival (3). Mammography is currently the routine examination used for postoperative follow-up. However, both radiation therapy and surgery can lead to changes in the breast that can be difficult to distinguish from a local recurrence. Furthermore, the findings may be subtle. Therefore, there has been significant interest in both adjunctive imaging strategies and the risk factors associated with recurrence.
In our current study, the IBTR group frequently displayed calcification on the mammography and a mass on the US. Calcification is well known to be easily detected and characterized by mammography; however, dense breast parenchymal tissue frequently obscures tumor masses on a mammography. In addition, USs have a crucial role in recurrent lesion detection, especially in patients that present with non-palpable chest wall or axillary lymph node recurrences (15). In our RLNR & CBC group, lymph node metastases, especially at the axillary lymph node, were more common than recurrence in the contralateral breast and could not be detected without US. Therefore, both mammography and US may be important in the postoperative follow-up of a breast-conserving operation. Although routine breast screening USs are not encouraged presently, several studies on US surveillance for the detection of regional lymph node recurrences after breast cancer surgery have concluded that lymph node US evaluations provide a useful means of detecting lymph node cancer recurrence in early stages in asymptomatic patients (151617).
Similar to previous studies (456101819), our present study results indicate that both the IBTR and RLNR & CBC groups showed greater tendencies toward a younger age; negative node status; higher nuclear and histologic grades; negative ER, PR, and HER-2 status; and basal-like subtype. Nixon et al. (5) reported that young patients have a greater percentage of tumors with an extensive intraductal component, higher degree of mononuclear inflammatory reaction, higher tumor grade, and lymphatic vessel invasion. The axillary nodal involvement seems to have no impact on local recurrence as shown in previous studies (49). In a study by Komoike et al. (18), young age, positive surgical margin, and omission of radiation therapy were found to be significant predictors for IBTR. Noguchi et al. (19) reported that the type of cancer and grade, presence of tumor emboli, endolymphatic invasion, ER-negativity, increased expression of HER-2, and p53-positivity are all variables associated with local recurrence risk. In addition, among the various markers that we investigated in our current analyses, ER negativity, HER-2 overexpression, and p53 positivity were more frequent in the IBTR group then in the RLNR & CBC group. Further studies are necessary to evaluate the difference and clinical implication of risk factors between IBTR, RLNR, and CBC.
Our study had several limitations. First, we did not consider whether breast radiation and neoadjuvant or adjuvant systemic therapy, which are significant predictors for IBTR, were added after breast-conserving surgery. Further prospective studies with a larger patient pool is necessary considering these radiation and systemic therapies. Second, we did not distinguish between the recurrence of the original tumor and a new primary tumor, which would have a different prognosis. However, ipsilateral second primary tumors are not treated differently from true recurrences because the therapy is based on molecular classification. Third, we compared only the IBTR and RLNR & CBC groups without a control group.
Finally, we did not distinguish between cancer recurrence and early treatment failure. Of 76 the recurring breast cancer cases, 15 recurred within one year after the initial treatment. Five cases were diagnosed as recurrence due to the newly detected calcifications and 10 other cases may be due to treatment failure. The clinical course and interval period time may classify the two different groups, however, some aggressive tumors may recur faster than we expect despite proper preoperative examination and additional early treatments.
In conclusion, a mass or calcification appears frequently in IBTR patients and the axillary lymph node is the most frequent site for RLNR. The ER and HER-2 status of the tumor are considered to be major independent factors associated with recurrent breast cancer. For patients with ER-negative and HER-2-overexpressing recurrent tumors, breast imaging follow-up should be strongly considered after breast-conserving surgery for invasive ductal carcinoma.
Figures and Tables
 | Fig. 1A 53-year-old woman underwent left breast-conserving surgery due to invasive ductal cancer 12 months prior. The left craniocaudal spot compression magnification view (A) shows grouped fine pleomorphic microcalcifications adjacent to the previous operation site (arrows). Ultrasonography (B) shows a 1.2-cm, low-echoic, ill-defined mass with intratumoral calcifications, proven to be a reccurent invasive ductal carcinoma by an ultrasonogram-guided core needle biopsy. |
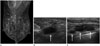 | Fig. 2A 36-year-old woman underwent right breast-conserving surgery due to invasive ductal cancer seven months prior. A mediolateral oblique mammography (A) demonstrates postoperative changes in both the right breast and axilla, without any abnormal postoperative findings. Ultrasonography was perfomed on the same day and shows multiple enlarged lymph nodes (arrows) at the right axilla level I (B) and II (C), proven to be metastatic lymph nodes by ultrasonogram-guided fine needle aspiration. |
Table 1
Comparison of Lesion Types on Mammography and Ultrasound between the IBTR and RLNR & CBC Groups

Table 2
Univariate Analysis of the IBTR and RLNR & CBC Groups

References
1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.
2. Jatoi I, Proschan MA. Randomized trials of breast-conserving therapy versus mastectomy for primary breast cancer: a pooled analysis of updated results. Am J Clin Oncol. 2005; 28:289–294.
3. Lu WL, Jansen L, Post WJ, Bonnema J, Van de Velde JC, De Bock GH. Impact on survival of early detection of isolated breast recurrences after the primary treatment for breast cancer: a meta-analysis. Breast Cancer Res Treat. 2009; 114:403–412.
4. Touboul E, Buffat L, Belkacémi Y, Lefranc JP, Uzan S, Lhuillier P, et al. Local recurrences and distant metastases after breast-conserving surgery and radiation therapy for early breast cancer. Int J Radiat Oncol Biol Phys. 1999; 43:25–38.
5. Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994; 12:888–894.
6. Kini VR, Vicini FA, Frazier R, Victor SJ, Wimbish K, Martinez AA. Mammographic, pathologic, and treatment-related factors associated with local recurrence in patients with early-stage breast cancer treated with breast conserving therapy. Int J Radiat Oncol Biol Phys. 1999; 43:341–346.
7. Kurtz JM, Jacquemier J, Amalric R, Brandone H, Ayme Y, Hans D, et al. Breast-conserving therapy for macroscopically multiple cancers. Ann Surg. 1990; 212:38–44.
8. Recht A, Come SE, Henderson IC, Gelman RS, Silver B, Hayes DF, et al. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996; 334:1356–1361.
9. Veronesi U, Marubini E, Del Vecchio M, Manzari A, Andreola S, Greco M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995; 87:19–27.
10. Magee B, Swindell R, Harris M, Banerjee SS. Prognostic factors for breast recurrence after conservative breast surgery and radiotherapy: results from a randomised trial. Radiother Oncol. 1996; 39:223–227.
11. Freedman GM, Fowble BL. Local recurrence after mastectomy or breast-conserving surgery and radiation. Oncology (Williston Park). 2000; 14:1561–1581. discussion 1581-1582, 1582-1584
12. Park CC, Mitsumori M, Nixon A, Recht A, Connolly J, Gelman R, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000; 18:1668–1675.
13. Khatcheressian JL, Wolff AC, Smith TJ, Grunfeld E, Muss HB, Vogel VG, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006; 24:5091–5097.
14. Francis M, Cakir B, Ung O, Gebski V, Boyages J. Prognosis after breast recurrence following conservative surgery and radiotherapy in patients with node-negative breast cancer. Br J Surg. 1999; 86:1556–1562.
15. Kim SJ, Moon WK, Cho N, Chang JM. The detection of recurrent breast cancer in patients with a history of breast cancer surgery: comparison of clinical breast examination, mammography and ultrasonography. Acta Radiol. 2011; 52:15–20.
16. Shin JH, Han BK, Choe YH, Nam SJ, Park W, Im YH. Ultrasonographic detection of occult cancer in patients after surgical therapy for breast cancer. J Ultrasound Med. 2005; 24:643–649.
17. Moon HJ, Kim MJ, Kim EK, Park BW, Youk JH, Kwak JY, et al. US surveillance of regional lymph node recurrence after breast cancer surgery. Radiology. 2009; 252:673–681.
18. Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006; 106:35–41.
19. Noguchi S, Koyama H, Kasugai T, Tsukuma H, Tsuji N, Tsuda H, et al. A case-control study on risk factors for local recurrences or distant metastases in breast cancer patients treated with breast-conserving surgery. Oncology. 1997; 54:468–474.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download