Abstract
Multicystic dysplastic kidney is a common cystic renal disease that often occurs in infancy. Recent studies demonstrate the possibility for spontaneous involution of a dysplastic kidney. In such cases, the prognosis is generally excellent and there is a very low incidence of complications. Complications associated with multicystic dysplastic kidney include pain, infection, hypertension, and neoplasia. Renal cell carcinomas are extremely rare in multicystic dysplastic kidneys. To our knowledge, no case report has described a radiologic finding of renal cell carcinoma arising from an involutional multicystic dysplastic kidney. We report a case of histopathologically validated cystic papillary renal cell carcinoma arising from an involutional multicystic dysplastic kidney and describe its sonographic and CT features.
Multicystic dysplastic kidney is the most common form of cystic kidney disease in infants and children. It can arise from failure of the ureteric bud to integrate and branch appropriately into the metanephros during development (1). The involvement is usually unilateral, although bilateral disease can also occur, and in some patients there is segmental involvement of one kidney. Most multicystic dysplastic kidneys are benign and do not require surgical resection (2). Reported complications associated with multicystic dysplastic kidney include pain, infection, hypertension, and neoplasia (3). However, renal cell carcinomas are extremely rare in multicystic dysplastic kidneys. We found only three cases of renal cell carcinoma in previously published reports (456). Little is known regarding the imaging characteristics of this condition. We report the sonographic and CT features of a histopathologically proven cystic papillary renal cell carcinoma arising from an involutional multicystic dysplastic kidney. Institutional Review Board approval with waived informed consent (number 2014-02-009) was obtained for this case report.
A 33-year-old male with no significant past medical history was referred for evaluation of a left retroperitoneal mass incidentally detected on ultrasonography performed at another medical institution. Findings from a physical examination were unremarkable. Laboratory examinations, including urine cytology, yielded results within normal limits. Ultrasonography of the abdomen showed a unilocular cystic mass 7 cm in diameter with multiple, mural nodules located in the left renal fossa (Fig. 1A). The left kidney was not visible. The patient underwent CT examination of the abdomen and pelvis (Somatom Sensation 16, Siemens Medical Solutions, Forchheim, Germany) before and after administration of IV contrast material (Ultravist 370, Bayer-Schering, Berlin, Germany). The mass had a primarily cystic nature with a discrete calcification in the wall which could be seen on the unenhanced image (Fig. 1B). Contrast-enhanced CT images showed peripheral soft-tissue mural nodules with mild enhancement ranging from a value of 21 Hounsfield units (HU) on the unenhanced CT image to 50 HU on the nephrographic phase obtained 90 seconds after contrast dye administration (Fig. 1C-E). There was neither lymphadenopathy nor renal vein involvement.
Because this lesion had enhancing soft-tissue mural nodules, we could not immediately rule out renal malignancy in an involutional multicystic dysplastic kidney or a cystic retroperitoneal tumor in a patient with left renal agenesis. Therefore, our patient underwent surgical exploration. Radical nephrectomy was performed without complications. Subsequent CT scanning and ultrasonography of the patient's abdomen and urine cytology were normal at a follow-up examination performed two years post-treatment.
The excised cystic mass measured 8.7 × 7 × 6.5 cm. The outer surface was smooth and showed an area of fibrous thickening. On cross-section, the cystic cavity was unilocular and filled with clear, serous fluid. The inner surface showed multiple foci consisting of tan, papillary excrescences (Fig. 2A). Papillary tumor tissue, visualized microscopically, consisted of low-grade, eosinophilic renal carcinoma cells (Fig. 2B). There were also immature tubule-like structures lined by flattened or cuboidal epithelial cells scattered in the thickened areas of the wall, which suggested dysplastic kidney (Fig. 2C).
Multicystic dysplastic kidney disease is relatively common and is estimated to occur in one in 4300 live births (7). In general, patients with multicystic dysplastic kidney disease present with a unilateral abdominal mass, and as only one kidney is involved, overall renal function is normal. In general, most multicystic dysplastic kidneys spontaneously involute or decrease in size over time and have low rates of complications and malignant degeneration. Therefore, such kidneys are for the most part currently left untreated, while in the past they were routinely removed (7).
Reported complications associated with multicystic dysplastic kidney include pain, infection, hypertension, and neoplasia (6). Until now, only three reports have been published that describe renal cell carcinomas arising from multicystic dysplastic kidneys (456). In one case, the renal cell carcinoma was a papillary adenocarcinoma; histologic subtype of the other two cases is undocumented. Two of the three patients were young (15 and 26 years old). Two had mainly cystic tumors with solid components. However, these patients had aggressive tumors with early metastases involving lymph nodes, bone, and lung. Our patient is unique in that he had a cystic renal mass containing mildly enhancing mural nodules in an involutional multicystic dysplastic kidney, which replaced the normal kidney, and was asymptomatic. To our knowledge, our case is the first radiology imaging report of a renal cell carcinoma in an involutional multicystic dysplastic kidney.
Papillary renal cell carcinoma is the second most common histological subtype, accounting for 10-15% of all renal cell carcinomas (8). As a papillary renal cell carcinoma is commonly a low-stage tumor, it is associated with a very good prognosis. The most important prognostic factors for patients with renal cell carcinoma are disease stage at the time of diagnosis and nuclear grade. A tumor is more likely to be cured by surgical resection when it is small and low grade. Therefore, early detection of renal cell carcinoma is crucial in order to improve patient survival rate. Cystic papillary renal cell carcinomas, such as that in our patient, demonstrate peripheral soft-tissue mural nodules that are enhanced by administration of contrast dye. They typically appear hypovascular and homogenous on contrast-enhanced CT.
Our current understanding of multicystic dysplastic kidney is incomplete. The incidence of malignant degeneration is also unknown. The majority of multicystic dysplastic kidneys are clinically benign; some cysts spontaneously decrease in size and subsequently regress. However, as shown in previous studies, dysplastic tissue may not completely disappear despite cyst shrinkage and fluid reabsorption (6), and the malignant potential of even small amounts of residual tissue is unknown. Our case demonstrates that there is a potential risk of malignant degeneration even in an involutional multicystic dysplastic kidney. Although the incidence of malignant degeneration is low, careful consideration of elective nephrectomy is advisable in such cases.
Figures and Tables
Fig. 1
33-year-old man with a left retroperitoneal mass.
A. Ultrasonogram of the left intercostal space shows a cystic mass with multiple, discrete nodularites (arrows) in the left renal fossa. However, the left kidney was not clearly visible.
B. Unenhanced CT image shows a cystic mass with a linear calcification.
C-E. Contrast-enhanced CT images obtained during the nephrographic phase show several focal nodular enhancements (long arrows) in the cyst wall. Note the triangular, regressed, multicystic dysplastic kidney (short arrows) between the adrenal gland and the cystic mass. The normal parenchyma of the left kidney is not visible.
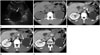
Fig. 2
Histopathologic findings of a surgically resected, left retroperitoneal mass.
A. The cut surface of the surgical specimen shows tan papillary tumor tissue on the inner surface of the wall.
B. Photomicrograph showing immature, tubule-like structures scattered in the fibrous cystic wall (arrows), which is a dysplastic kidney (hematoxylin and eosin stain, × 100).
C. Photomicrograph showing low-grade renal cell carcinoma with papillary arrangement (arrows) (hematoxylin and eosin stain, × 40).

Acknowledgments
We thank Bonnie Hami, MA (USA) for her editorial assistance in preparing the manuscript.
References
1. Matsell DG, Bennett T, Goodyer P, Goodyer C, Han VK. The pathogenesis of multicystic dysplastic kidney disease: insights from the study of fetal kidneys. Lab Invest. 1996; 74:883–893.
2. Weinstein A, Goodman TR, Iragorri S. Simple multicystic dysplastic kidney disease: end points for subspecialty follow-up. Pediatr Nephrol. 2008; 23:111–116.
3. Cambio AJ, Evans CP. Outcomes and quality of life issues in the pharmacological management of benign prostatic hyperplasia (BPH). Ther Clin Risk Manag. 2007; 3:181–196.
4. Barrett DM, Wineland RE. Renal cell carcinoma in multicystic dysplastic kidney. Urology. 1980; 15:152–154.
5. Birken G, King D, Vane D, Lloyd T. Renal cell carcinoma arising in a multicystic dysplastic kidney. J Pediatr Surg. 1985; 20:619–621.
6. Rackley RR, Angermeier KW, Levin H, Pontes JE, Kay R. Renal cell carcinoma arising in a regressed multicystic dysplastic kidney. J Urol. 1994; 152(5 Pt 1):1543–1545.
7. Cambio AJ, Evans CP, Kurzrock EA. Non-surgical management of multicystic dysplastic kidney. BJU Int. 2008; 101:804–808.
8. Prasad SR, Humphrey PA, Catena JR, Narra VR, Srigley JR, Cortez AD, et al. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics. 2006; 26:1795–1806. discussion 1806-1810




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download