Abstract
Fungal infections occur in severely immunocompromised patients having profound and prolonged neutropenia. Here, we report a case of a 41-year-old female who, at the conclusion of induction chemotherapy for acute T-lymphoblastic leukemia, developed angioinvasive mucormycosis involving the appendix and liver, which presented as abdominal pain. This case is the first to provide detailed computed tomography and magnetic resonance imaging findings of angioinvasive appendiceal and hepatic mucormycosis. The implications of these findings as well as the diagnosis and management of mucormycosis, is further discussed.
Mucormycosis is a rare invasive and fatal fungal infection occurring most frequently in patients with immune deficiencies and diabetes mellitus. It refers to several different diseases caused by fungi of the class Zygomycetes (1). As the incidence of invasive fungal infection has increased in patients with hematologic malignancies and in transplant recipients, the occurrence of mucormycosis has risen, and it is now recognized as the third most common cause of fungal infections, after candidiasis and aspergillosis (2). The most common manifestations of mucormycosis are rhinocerebral and pulmonary involvement. Here, we present the imaging findings of disseminated mucormycosis with extensive involvement of the liver and appendix. This case study was approved by the Institutional Review Board, and the need for a written informed consent was waived.
A 41-year-old female visited our hospital with palpable inguinal masses. On physical examination, she had a mild fever with bilateral cervical and inguinal lymphadenopathies. The lesions were excised surgically, and diagnosed as acute T-cell lymphoblastic leukemia. The patient was started on intensive induction chemotherapy using the hyper-CVAD regimen (fractionated cyclophosphamide, vincristine, adriamycin, dexamethasone). When the patient was in the severe neutropenic period after four cycles of chemotherapy, defined as an absolute neutrophil count < 0.5 × 109/L, she complained of vague abdominal pain. The next day, she presented with decreased mental alertness and more intensified abdominal pain. An urgent computed tomography (CT) scan of the abdomen showed multiple, variable-sized, hypodense lesions in both the hepatic lobes, without significant enhancement. Decreased mural enhancement of the appendix with mild edematous wall thickening was also observed (Fig. 1A). We predicted a high probability of hepatic and appendiceal infarctions by angioinvasive fungal infection, and immediately started her on intravenous amphotericin B deoxycholate (1.9 mg/kg/day) along with broad-spectrum antibiotics. On a follow-up abdominal CT scan 2 weeks later, the hepatic lesions showed clearer margins with peripheral enhancement. Hepatic and appendiceal lesions extending to the fat beyond the involved organs were confined by enhancing rim-like lesions (Fig. 1B). MR imaging was performed by using a 3-T system (Signa HDx, GE Healthcare, Milwaukee, WI, USA) with contrast enhancement (Primovist; Bayer-Schering, Berlin, Germany). Multiple hepatic lesions showed internal T2 high signal intensity (SI) with peripheral intermediate SI rims in both hepatic lobes. On dynamic enhanced images, the center of these lesions were not found to be enhanced, but peripheral rims were progressively enhanced (Fig. 1C-F). Despite continuous medical treatment for two months, the extent of the hepatic lesions hardly improved, and the infarcted appendix atrophied. With improvement in the patient's general condition, surgical debridement, along with excision of the hepatic lesions with a cecectomy, was performed. Gomori Methenamie-Silver and hematoxylineosin staining of the excised hepatic specimens revealed large areas of coagulative necrosis surrounded by thick collagenous fibrosis and karyorrhectic debris. Many fungal organisms compatible with mucormycosis were found in the coagulative necrosis (Fig. 1G, H). Innumerous hyphae were also seen within the adjacent intrahepatic vascular lumens. However, the appendix was so severely atrophied that the pathological examination did not reveal any demonstrable hyphae in the cecectomy specimen. The patient remained hospitalized and underwent consolidative chemotherapy combined with persistent amphotericin B therapy, for approximately 4 months after the first onset of symptoms.
Mucormycosis is a rare, invasive fungal infection, most frequently seen in immunocompromised patients, particularly during the neutropenic period. The rate of invasive fungal infection is strongly correlated with the type of cytotoxic agents and the duration of bone marrow aplasia (3).
Mucormycosis can manifest as rhinocerebral, pulmonary, cutaneous, gastrointestinal, disseminated, and miscellaneous infections. Gastrointestinal involvement is a rare form, representing 3-7% of all mucormycosis cases. The stomach is the most frequently involved (57.5%), followed by the colon (32.3%) and ileum (6.9%) (4). Hepatic involvement is rare, and commonly presents in a patient with pulmonary or gastrointestinal infection; therefore, it is considered a disseminated disease (5). The main route of transmission is not clear in the present case, although the initial images suggested appendiceal and hepatic involvement, and there was no radiological or clinical evidence of any other source of infection. Thus, we concluded that the appendiceal infection disseminated to the liver.
The imaging findings of gastrointestinal and hepatic mucormycosis have rarely been reported or described in radiological literature. Early CT findings of gastrointestinal involvement of angioinvasive fungi have noted nonspecific bowel wall thickening with decreased mural enhancement. As the infection progresses, the involved bowel segment shows no enhancement, suggesting necrosis, and the bowel wall thins or even disappears (6). In the liver, multiple hypodense hepatic lesions surrounding vascular structures without a mass effect suggests an angioinvasive organism, resulting in a thrombosed occlusion by fungi and subsequent necrosis of the perivascular hepatic tissue. Although imaging findings are not pathognomonic, they can play a decisive role in a suspicious clinical setting of angioinvasive fungal infection, including mucormycosis (6).
The prognosis of mucormycosis remains extremely poor, with a mortality rate > 40%, despite aggressive medical and surgical treatments. In particular, patients with hematologic malignancy or recipients of hematopoietic stem cell transplantation, have mortality rates of up to 65% and 90%, respectively (78). Of all the forms of mucormycosis, disseminated mucormycosis has the highest mortality, and is frequently diagnosed ante mortem (6). In our case, the patient was in relatively good general condition, was still alive 6 months after this episode, and has since been re-hospitalized for re-induction chemotherapy with sustained antifungal therapy.
Prompt diagnosis of mucormycosis is critical for survival; any delay can be fatal. Diagnosis of mucormycosis or any other fungal infection is usually difficult at an early stage, because its nonspecific presentation requires a high degree of suspicion. There can be various presumptive diagnoses in a patient who complains of abdominal pain, after chemotherapy treatment or in any other immunocompromised state. In addition, a chemotherapy agent can mask the typical symptoms and signs, thus further delaying diagnosis. The standard therapy for mucormycosis is a combination of medical and surgical treatments. Correction of underlying disease and any reversible predisposing factor is an important feature for the outcome of infection. In neutropenic patients, recovery from neutropenia is strongly correlated with better outcome and clinical improvement. If the neutropenic period is not terminated, antifungal agents are ineffective (9). Lee et al. (10) reported a similar case of intestinal and hepatic mucormycosis in a patient with acute lymphoblastic leukemia, who succumbed to progressive liver failure despite undergoing intestinal surgery and receiving antifungal treatment.
In summary, we report a case of appendiceal and hepatic mucormycosis presenting as abdominal pain, in a neutropenic individual with acute lymphoblastic leukemia. Poorly enhancing lesions extended to the surrounding fat tissue beyond the involved organs, and peripheral enhancing thin rims confined the lesions in the liver and appendix.
Figures and Tables
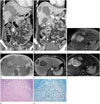
Fig. 1
A 41-year-old female with appendiceal and hepatic mucormycosis.
A. Coronal image: multiple, variable-sized, non-enhancing hypodense lesions are seen in the liver. At same time, the appendix shows decreased mural enhancement (arrow) with mild periappendiceal fat infiltration.
B. Follow-up CT scan obtained 2 weeks later: hepatic lesions show clearer margins with peripheral enhancement. Hepatic and appendiceal lesions extending to the fat beyond the involved organs are confined by enhancing rim-like lesions (arrowheads).
C-F. Liver dynamic MRI: an axial T2-weighted image shows high signal intensity (SI) hepatic lesions surrounded with intermediate SI rims (C). Peripheral thick rims show slightly low SI on T1-weighted image, while inner portion of lesions show high SI (D). Peripheral enhancing rims on portal phase scan (E) are correlated with high SI on high b-value (800 s/mm2) diffusion weighted image (F).
G. The hepatic parenchyma shows extensive necrosis surrounded by karyorrhectic debris and dense collagenous fibrosis (hematoxylin-eosin stain, × 100). Many fungal hyphae are noted in the necrosis.
H. Gomori Methenamie-Silver (GMS) stain highlights broad, nonseptated hyphae, consistent with mycormycosis (GMS, × 400).
References
1. Kontoyiannis DP, Lewis RE. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am. 2006; 20:581–607. vi
2. Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Piccardi M, et al. Mucormycosis in hematologic patients. Haematologica. 2004; 89:207–214.
3. Bow EJ, Loewen R, Cheang MS, Schacter B. Invasive fungal disease in adults undergoing remission-induction therapy for acute myeloid leukemia: the pathogenetic role of the antileukemic regimen. Clin Infect Dis. 1995; 21:361–369.
4. Thomson SR, Bade PG, Taams M, Chrystal V. Gastrointestinal mucormycosis. Br J Surg. 1991; 78:952–954.
5. Suh IW, Park CS, Lee MS, Lee JH, Chang MS, Woo JH, et al. Hepatic and small bowel mucormycosis after chemotherapy in a patient with acute lymphocytic leukemia. J Korean Med Sci. 2000; 15:351–354.
6. Hagspiel KD, Kempf W, Hailemariam S, Marincek B. Mucormycosis of the liver: CT findings. AJR Am J Roentgenol. 1995; 165:340–342.
7. Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005; 41:634–653.
8. Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases. Leuk Lymphoma. 2004; 45:1351–1360.
9. Antman KS, Griffin JD, Elias A, Socinski MA, Ryan L, Cannistra SA, et al. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on chemotherapy-induced myelosuppression. N Engl J Med. 1988; 319:593–598.
10. Lee JH, Ha HK, Yoo E, Yang SK, Min YI, Auh YH. CT and sonographically guided biopsy in a patient with intestinal mucormycosis. AJR Am J Roentgenol. 2000; 175:129–131.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download