Abstract
Osteofibrous dysplasia (OFD) is a benign fibro-osseous lesion found in long bones, and congenital OFD in neonates is very rare. The diagnosis of OFD in neonates is difficult, and it is sometimes misidentified as any of a number of other congenital tumors or tumor-like lesions, in which case biopsies are often necessary. After a histological confirmation of OFD, non-surgical or delayed surgical treatment is generally recommended. We present image findings from the radiographs and magnetic resonance images in the case of a 7-day-old female infant with pathologically confirmed congenital OFD.
Osteofibrous dysplasia (OFD) is a benign fibro-osseous lesion found in long bones, frequently affecting the tibia of children (12). Congenital OFD is rare, and only a few cases have been reported. A diagnosis of congenital OFD is difficult, due to its rarity and the absence of characteristic radiographic features. We, herein, present the case of a female infant with congenital OFD that presented with aggressive imaging features.
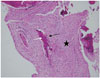
A 7-day-old female infant was admitted to our hospital for an evaluation of swelling in the right lower leg. She had no significant birth complications. Radiographs demonstrated an expansile osteolytic lesion with inner irregular sclerosis, involving the cortex and medullary cavity of the proximal meta-diaphysis of the right tibia. The margin of the lesion was irregular, multi-lobulated, but relatively well-defined. The cortical disruption of the proximal tibia suggested a pathologic fracture, and a thin linear periosteal reaction at its distal part was noted (Fig. 1). Magnetic resonance (MR) images showed a mass replacing the entire medullary space, which revealed heterogeneous hyperintense signal intensity on the T2-weighted image and intermediate signal intensity on the T1-weighted image, as well as heterogeneous enhancement on the Gd-enhanced T1-weighted image. A disrupted periosteal reaction continuous to the pathologic fracture and prominent signal change in the extraosseous soft tissue were noted, and the transition between the extraosseous component and the normal muscles was wide and ill-defined (Fig. 2). The differential diagnoses of this lesion comprised a number of congenital diseases (including malignancy), and a subsequent biopsy was recommended. A surgical-excision biopsy on a portion of the lesion was performed, and the histological result showed fibrous stromas with immature bony trabeculae and osteoblastic rim. Subsequently, the final diagnosis of congenital OFD was made (Fig. 3). Follow-up radiographs (after 3 weeks) showed a callus formation at the pathologic fracture, and a bowing deformity of the proximal tibia. On this image an osteolytic lesion, that was not detected on initial radiograph, was additionally found in the distal dia-metaphysis of the fibula. Although the lesion was also presumed to be OFD, MR images for this lesion were not obtained (Fig. 1).
The term OFD, which was first described by Campanacci in 1976, appeared formerly in literature under "ossifying fibroma"-subsequent authors have preferred the term OFD (23). The age range of patients typically affected by OFD is seven days to 22 years, with neonatal cases being very rare. Consistent with other infantile bone lesions, the diagnosis of congenital OFD is especially difficult. The clinical presentation is usually marked by swelling or bowing of the leg with pain (3).
The pathogenesis of OFD is not well-known. It has been postulated that OFD results from excessive resorption of bone, with a subsequent fibrous repair of the defect (4). The histological appearance of OFD is characterized by a fibrous matrix with numerous irregularly shaped trabeculae of woven bone that are often rimmed by osteoblasts. Histologically, the main differential diagnoses are differentiated adamantinoma and fibrous dysplasia. Aside from its preferential involvement with the tibia, OFD has many overlapping clinical, morphologic and pathological similarities with differentiated adamantinoma; some authors have hypothesized that OFD and adamantinoma may simply be different stages of the same disease, but the relationship between the two remains controversial (25). Fibrous dysplasia typically originates in the intramedullary space rather than in the cortex, and bowing of the involved bone is rarely combined. Histologically, fibrous dysplasia has more cellular and less-mature fibrous tissue than OFD. In fibrous dysplasia, osteoblastic rimming of bone trabeculae may be composed of lamellar rather than of woven bone (3).
The common radiographic finding of OFD in children is well-defined eccentric osteolytic lesions involving the metaphysis of long bones, with consequent cortical expansion and bowing of the bone in the antero-posterior direction. On the MR image, OFD reveals intermediate to high signal intensity on the T2-weighted image and intermediate signal intensity on the T1-weighted image, which may be similar to other tumors with fibroblastic stroma. Superimposed hemorrhagic, cystic, myxoid change and cartilaginous differentiation can contribute to heterogeneous signal intensity on the T2-weighted image. Cortical breakage and perilesional soft tissue extension can be combined, which is mostly associated with pathologic fracture (5). According to previous literature, it seems that congenital OFD can more frequently be associated with pathologic fracture and pseudoarthrosis and, reveal more prominent perilesional reactive change (26). Thus, although OFD is a benign, slowly-progressive lesion, sometimes it can mimic other progressive tumors (or tumor-like lesions) such as Langerhans-cell histiocytosis (LCH), myofibromatosis, neurofibromatosis, infection-related lesions, and malignant tumors, especially in neonates (13). Most of these are difficult to differentiate by their clinical or radiographic appearance, in which case biopsy is often necessary.
LCH, most commonly occurring in children, demonstrates a broad spectrum of clinical and radiologic features that can mimic those of infection, benign and malignant tumors. During the early phase, there is a more-aggressive pattern of osteolysis reflective of the biologic activity. Localized LCH commonly occurs in patients of 5–15 years of age; younger patients under 2 years old are usually afflicted with disseminated multi-system involvement, which is known as Letterer-Siwe disease (7). As for congenital infantile myofibromatosis, it is a rare, potentially OFD-mimicking disorder. Common findings of bone lesions are well-defined lytic lesions with, or without, sclerotic borders. The typical clinical course of the solitary form is initial rapid growth followed by spontaneous regression within the first two years. However, infantile myofibromatosis more commonly presents as the multi-centric type (8). Neurofibromatosis has been reported in 40–80% of patients suffering from congenital pseudoarthrosis of the tibia, whose radiographic appearance can mimic congenital OFD. However, neurofibromatosis mainly involves the periostium of the long bones; the medullary canal, unlike in congenital OFD, usually is narrowed by a thickening of the cortical bone (9). As for infection, neonatal osteomyelitis is characterized by destructive lesions with abundant periosteal reaction in the metaphysis. It usually shows prompt articular involvement and various clinical signs, such as fever, leukocytosis and joint swelling (10). Finally, as an example of a malignant tumor, Ewing's sarcoma should be considered. In infants, Ewing's sarcoma (due to its rarity) is often misdiagnosed as either a fracture or an infection. The typical radiographic appearance of Ewing's sarcoma in the long bones is a permeative lesion with a lamellar "onion-skin" periosteal reaction.
The treatment of OFD is controversial. OFD usually shows a slow progression and stabilizing after skeletal maturity; however, spontaneous regression of congenital OFD has been described in a few cases. Jobke et al. (1) reported a case with spontaneous post-biopsy regression, positing a potential for self-healing and remodeling at very young ages, and speculated that biopsy-incurred alteration of the lesion comparable to the effect of a spontaneous fracture through it can initiate healing in parallel with physiological bone maturation and diaphyseal growth. Though some authors have advocated radical surgery, others have emphasized that surgery should be delayed as long as possible, and restricted to extensive lesions or to cases of combined pathological fracture (2).
In conclusion, congenital OFD is a benign, slowly-progressive lesion frequently affecting the tibia. Sometimes, it can present with aggressive imaging features such as complete intramedullary involvement, pathologic fracture with prominent perile-sional reactive change and even pseudoarthrosis. Although OFD is very rare in neonates, it should be considered in differential diagnoses of congenital tumor or tumor-like lesions in the lower limbs.
Figures and Tables
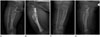 | Fig. 1Radiographs.
A, B. The initial AP and lateral image demonstrate an expansile osteolytic lesion (black arrow) with inner irregular sclerosis, involving the cortex and medullary cavity of the proximal meta-diaphysis of the right tibia. The margin of the lesion is irregular, multi-lobulated, but relatively well-defined. The cortical disruption of the proximal tibia suggests a pathologic fracture (white arrow), and a thin linear periosteal reaction at its distal part is noted.
C, D. The follow-up AP and lateral image (after 3 weeks) show a callus formation at the pathologic fracture, and a bowing deformity of the proximal tibia. An osteolytic lesion (arrowhead), not detected on the initial radiograph, is seen in the distal dia-metaphysis of the fibula.
|
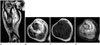 | Fig. 2MRI of the right lower leg.
A. A sagittal T2-weighted MR image shows an expansile mass replacing the entire medullary space, which reveals heterogeneous hyperintense signal intensity (white arrow). A disrupted periosteal reaction, continuous to the pathologic fracture, is noted (black arrow).
B-D. The axial T2-weighted (B), T1-weighted (C), and enhanced T1-weighted (D) MR images show prominent signal change with heterogeneous enhancement in extraosseous soft tissue (star). The transition between the extraosseous component and the normal muscles is wide and ill-defined.
|
References
1. Jobke B, Bohndorf K, Vieth V, Werner M. Congenital osteofibrous dysplasia Campanacci: spontaneous postbioptic regression. J Pediatr Hematol Oncol. 2014; 36:249–252.
2. Zamzam MM. Congenital osteofibrous dysplasia of the tibia, associated with pseudoarthrosis of the ipsilateral fibula. Saudi Med J. 2008; 29:1507–1509.
3. Smith NM, Byard RW, Foster B, Morris L, Clark B, Bourne AJ. Congenital ossifying fibroma (osteofibrous dysplasia) of the tibia--a case report. Pediatr Radiol. 1991; 21:449–451.
4. Hahn SB, Kang ES, Jahng JS, Park BM, Choi JC. Ossifying fibroma. Yonsei Med J. 1991; 32:347–355.
5. Jung JY, Jee WH, Hong SH, Kang HS, Chung HW, Ryu KN, et al. MR findings of the osteofibrous dysplasia. Korean J Radiol. 2014; 15:114–122.
6. Teo HE, Peh WC, Akhilesh M, Tan SB, Ishida T. Congenital osteofibrous dysplasia associated with pseudoarthrosis of the tibia and fibula. Skeletal Radiol. 2007; 36:Suppl 1. S7–S14.
7. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992; 12:801–823.
8. Ang P, Tay YK, Walford NQ. Infantile myofibromatosis: a case report and review of the literature. Cutis. 2004; 73:229–231.
9. Maffulli N, Fixsen JA. Pseudoarthrosis of the ulna in neurofibromatosis. A report of four cases. Arch Orthop Trauma Surg. 1991; 110:204–207.
10. Kozlowski K, Beluffi G, Cohen DH, Padovani J, Tamaela L, Azouz M, et al. Primary bone tumours in infants. Short literature review and report of 10 cases. Pediatr Radiol. 1985; 15:359–367.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download