Abstract
Purpose
To determine whether benign and malignant bone tumors with associated pathologic fractures can be differentiated using radiologic findings.
Materials and Methods
Seventy-eight patients (47 men and 31 women, age range: 1-93 years) with a bone tumor and an associated pathologic fracture from 2004 to 2013 constituted the retrospective study cohort. The tumor size, margin, and enhancement patterns; the presence of sclerotic margin, the peritumoral bone marrow, soft tissue edema, extra-osseous soft tissue mass, intratumoral cystic/hemorrhagic/necrotic regions, mineralization/sclerotic regions, periosteal reaction and its appearance; and cortical change and its appearance were evaluated on all images. Differences between the imaging characteristics of malignant and benign pathologic fractures were compared using Pearson's chi-square test and the 2-sample t-test.
Results
There were 22 benign and 56 malignant bone tumors. Some factors were found to significantly differentiate between benign and malignant tumors; specifically, ill-defined tumor margin, the presence of sclerotic tumor margin and an extra-osseous soft tissue mass, the absence of cystic/necrotic/hemorrhagic portions in a mass, the homogeneous enhancement pattern, and the presence of a displaced fracture and of underlying cortical change were suggestive of malignant pathologic fractures.
Pathologic fracture is a term used to describe a fracture occurring in a weakened bone after trivial injury (12). Although there are many causes of bone weakening, such as, tumors, infections, and some inherited bone disorders, pathologic fractures are usually associated with bone tumors. The differentiation between malignant and benign fractures may be difficult, particularly in elderly patients, who commonly suffer from osteoporotic fractures and benign tumors that may be locally aggressive (3). Moreover, extensive edema and hematoma around the fracture site makes it difficult to differentiate between benign and malignant bone tumors.
The most accurate diagnostic technique is tissue biopsy followed by pathologic examination; however, because of the healing process, it may be difficult to distinguish tissue from a needle biopsy of a pathologic fracture and osteogenic sarcoma (4). Furthermore, pathologic diagnoses based on needle biopsy may vary by biopsy site, and differ from final diagnoses after surgery. Thus, information obtained by needle biopsy before surgery is limited.
Conventionally, plain radiography is used routinely to detect and evaluate bone tumors, although its abilities to evaluate extra-osseous extension and characteristic components of tumors are limited (5). Computed tomography (CT) scans are particularly useful for detecting bone mineralization, and characterizing pathologic fractures. Magnetic resonance imaging (MRI) can be used to characterize lesions further in terms of tumor extent and extra-osseous extension. In fact, most bone lesions can be diagnosed correctly based on their radiological appearances, and location in bone (6). However, the differentiation of malignancy and benignity may be difficult in the presence of fractures.
In the current study, we aimed to determine whether benign and malignant bone tumors with an associated pathologic fracture could be differentiated using radiologic findings.
This study was approved by the Institutional Review Board of our institution. Requirement for informed consent from patients was waived, because of the retrospective nature of the study.
Bone tumor cases were identified from the electronic archives of MRI examinations of the musculoskeletal system using the search term "pathologic fracture". As a result, 154 cases were identified. However, 76 cases were excluded due to infectious or metabolic conditions, such as, osteomyelitis or osteomalacia, multiple bone masses at the fracture site, cortical disruption by soft-tissue masses or an expansile bony masses (impending fracture), or a bone tumor of diffuse form affecting entire long or short bones. All patients included in this study underwent surgery, and histological findings were obtained. Accordingly, 78 patients (47 men and 31 women, age range: 1-93 years, mean age: 49 years) were enrolled in the present study.
All patients underwent plain radiography and CT due to the suspicion of fracture, and all underwent MRI for the evaluation of a space-occupying lesion at the fracture site. MRI was performed at the fracture site. MRI was performed with a field strength of 1.5-3T using a variety of systems. All 78 patients underwent T1- and T2-weighted sequences with variable imaging planes, and gadolinium-enhanced imaging. Fat-suppressed T2-weighted sequences were obtained in all cases. Contrast-enhanced images, obtained using the fat-suppression technique, were also available for all patients.
Images were reviewed by 2 experienced musculoskeletal radiologists (with 3 and 12 years of experience, respectively) who were unaware of either clinical histories or pathologic diagnoses by consensus.
Tumor margins were evaluated on both CT and MR images. Tumor margins were classified as well- or ill-defined. The later was defined as any indistinct tumor margin. However, margins were investigated around fracture sites, because hematoma formation and edema hindered the assessment of tumor margins at fracture sites. Fracture type was classified as displaced or non-displaced based on plain radiography, CT, and MRI findings.
For imaging analysis, the following characteristics were considered on MR images; 1) tumor; longest diameter, 2) mass margin (well- or ill-defined), 3) the presence of peritumoral bone marrow or soft tissue edema on fat-suppressed T2-weighted or contrast-enhanced images, 4) the presence of an extra-osseous soft tissue mass, 5) the presence of the intra-tumoral cystic/hemorrhagic/necrotic portions, and 6) the enhancement pattern including heterogeneous, homogeneous, and peripheral rim, or septal enhancement. Margin definition was also assessed when an extra-osseous soft tissue mass was present.
We evaluated the following on CT scans and plain radiographs: 1) mass margin (well- or ill-defined), 2) the presence of a sclerotic margin, matrix mineralization, or sclerotic portions, 3) the presence of periosteal reaction and its pattern (not apparent, benign nature, including a single layered non-interrupted pattern, malignant nature, including Codman's triangle, sunburst, hair-on-end, and onion-like patterns), and 4) the presence and pattern of cortical change around the fracture site (not apparent, endosteal scalloping due to an expansile nature, or a permeative or 'moth-eaten' appearance).
Matrix mineralization and sclerosis were assessed based on presence or absence, and not by pattern or extent.
Data were analyzed using IBM SPSS statistics for windows, version 21 (IBM, Armonk, NY, USA). The significance of differences in the imaging characteristics of malignant and benign bone tumors were determined using Pearson's chi-square test for categorized variables, and the significance of age and size were determined using the 2-sample t-test.
Statistical significance was accepted for p values of < 0.05.
The 78 patients had 22 benign tumors and 56 malignant tumors (types, locations, and numbers of bone tumors were summarized in Table 1). The common types of benign bone tumors were giant cell tumor (GCT), aneurysmal bone cyst (ABC), and fibrous dysplasia and the most common malignant type was metastasis. Lung cancer was the most frequent primary cause of metastases (18/51, 35%), followed by hepatocelluar carcinoma (10/51, 19%), breast cancer (4/51, 8%), head and neck cancer (4/51, 8%), gastrointestinal cancer (4/51, 8%), prostatic cancer (3/51, 6%), thyroid cancer (2/51, 4%), bladder cancer (2/51, 4%), cervical cancer (1/51, 2%), renal cell carcinoma (1/51, 2%), cholangiocarcinoma (1/51, 2%), and liposarcoma (1/51, 2%). In the 78 patients, the involved sites were femur (n = 38), humerus (n = 30), tibia (n = 4), clavicle (n = 2), hand (n = 1), radius (n = 1), ulna (n = 1), and fibula (n = 1).
Image analyses findings of benign and malignant pathologic fractures were shown in Table 2. Tumor margin, enhancement pattern, and the presence of a sclerotic margin, extra-osseous soft tissue mass, cystic/necrotic/hemorrhagic portions within the mass, displaced fracture, and underlying cortical change were found to significantly differentiate benign and malignant bone tumors. Tumor size was not related to its benign/malignant status; benign tumors ranged from 2.5 to 12.9 cm (mean; 6.3 cm) and malignant tumors were from 1.3 to 26.8 cm (mean; 7.2 cm).
In cases with a benign pathologic fracture, the more frequently observed radiologic findings included a well-defined tumor margin with a sclerotic rim, no extra-osseous soft tissue mass, more cystic/necrotic/hemorrhagic portions within masses, peripheral rim enhancement or heterogeneous enhancement, underlying cortical change including endosteal scalloping, and a non-displaced fracture (Fig. 1). In contrast, in malignant pathologic fractures the prominently observed radiologic findings included ill-defined margins, the presence of extra-osseous soft-tissue mass, more homogeneous enhancement, and a displaced fracture (Fig. 2). Although benign and malignant pathologic fractures showed no significant gender effect (p = 0.37), they exhibited a significant age effect (cutoff value ≤ 35; sensitivity 100%, specificity 96.4%, p < 0.0001).
Soft tissue edema was observed in all 78 patients. An extra-osseous soft tissue mass was present in 13 patients with a malignant tumor; ten tumors had an ill-defined margin, and the other 3 showed a well-defined soft tissue mass that arose from a bony mass. For mineralization and sclerosis, 1 patient with a malignant bone tumor exhibited calcification within the mass, and 5 showed sclerosis. For periosteal reaction, 1 case with a benign bone tumor showed a single layered pattern, representing benign nature, and all 10 cases with malignant tumors showed an aggressive pattern, such as, hair-on-end, Codman's triangle, or sunburst types (Fig. 3).
Regarding tumor-induced cortical changes, all 22 benign bone tumors, except for 1, presented endosteal scalloping due to an expansile nature away from the fracture site, and 3 of 39 malignant bone tumors with underlying cortical changes showed endosteal scalloping with the other 36 exhibiting 'moth-eaten' or permeative appearance.
Sarcoma is commonly associated with pathologic fracture, regardless of age, and this association is often related to a serious pathology (78). In our cohort, most pathologic fractures were associated with malignant bone tumors (56/78, 72%), especially metastatic bone tumors (51/78, 65%). Pathological fracture of long bones is a common complication of metastatic disease with an incidence of -10% (91011), but it can also occur in association with benign bone tumors. Therefore, potentially benign aggressive or malignant processes should be biopsied after conducting clinical, radiological, and laboratory evaluations (2). However, bone tumor biopsy has its limitations, which are mainly related to the method, and to the availability of an experienced musculoskeletal pathologist to correctly interpret findings.
Poorly-defined margins, tumor extension from marrow through sites of cortical destruction, and irregular contrast enhancement with zones of necrosis are commonly associated with malignant bone tumors (12). However, Bui et al. (13) suggested that deep endosteal scalloping and even cortical penetration are not signs of malignancy in eccentrically located bone tumors. Thus, the differentiation between benign and malignant fractures is relatively complex.
In the present study, margin definition and the presence of a sclerotic tumor margin were significant factors for differentiating between benign and malignant bone tumors. Only 1 benign bone tumor (ABC) had an ill-defined margin, and 1 malignant tumor (clear cell chondrosarcoma) had a well-defined margin. Thus, when a portion of the clear outline of a lesion is ill-defined, and the abrupt border separating the lesion from adjacent normal bone is lost, increased biologic activity or even malignant progression should be expected (14). On the other hand, well-defined margins with a geographic destructive pattern usually indicate benign or slow-growing malignant nature (15).
The presence of an extra-osseous soft tissue mass was also found to significantly differentiate between benign and malignant bone tumors. Benign tumors and tumor-like lesions usually exhibit no soft tissue spread, with the exceptions of GCT and ABC (1416). However, we found no extra-osseous mass in the benign cases included in the study, even in cases of GCT (n = 2) and ABC (n = 6). On the other hand, 14 of the 56 malignant tumors had extra-osseous soft tissue mass, and of these, 4 had a well-defined margin and 10 an ill-defined margin. These findings were in contrast with those of a previous report, in which malignant tumor masses were described as clear and spread through destroyed cortex while sparing tissue planes (14).
In the present study, homogeneous enhancement and the absence of a cystic/necrotic/hemorrhagic portion were significantly more common among malignant bone tumors. This finding agrees with that a histologic study, in which > 90% necrosis in a resected specimen of osteosarcoma was associated with a favorable outcome (1). Therefore, the lack of a necrotic portion and increased cellularity (more homogeneous enhancement) might increase the possibility of malignancy. However, in the present study, a larger cystic/necrotic/hemorrhagic portion showed an increased risk of fracture for benign bone tumors.
Displaced fracture was significantly more common in malignant bone tumors (18/56, 32% vs. 1/22, 4%), indicating that the pattern of pathologic fracture is more aggressive in malignant bone tumors.
The study results indicated that endosteal scalloping was common for benign bone tumors (21/22, 95%), whereas a permeative or 'moth-eaten' pattern was more common for malignant bone tumors (36/58, 62%). In a study by Bui et al. (13), scalloping was not associated with biological activity, growth, or malignancy. However, cortical disruption with a permeative pattern on MR images could result from a rapidly growing, more aggressive lesion. Therefore, patterns of underlying cortical changes caused by tumors even in the presence of a pathologic fracture might aid in the differentiation between benign and malignant pathologic fractures.
In the present study, the presence of mineralization or sclerosis with periosteal reaction was not significantly associated with benign/malignant tumor status. However, 10 malignant bone tumors showed aggressive periosteal reactions. Despite the non-specificity for a particular lesion, these reactions indicated a relationship between a more interrupted and complex pattern and greater biologic activity suggestive of lesions more likely to be aggressive (14). Periosteal reactions to malignant neoplasms can be identified by "hair-on-end" or "sunburst" patterns or as multilayered zones, such as, "onion-peel" or Codman's triangles (12). When periosteal reaction is evident, malignant and benign pathologic fractures were accurately differentiated in the present study, as previously reported.
Sex, tumor size, and peritumoral bone marrow edema were not significant differentiating factors between benign and malignant bone tumors. Peritumoral bone marrow and soft tissue edema may develop as reactive changes to bone fracture or cortical disruption. Furthermore, the presence of edema can make tumor characterization difficult, and thus, hinder differentiation. Even for benign bone tumors, especially bone tumors with an expansile nature, such as, non-ossifying fibroma, GCT, ABC, or fibrous dysplasia, the larger lesion size has a greater likelihood of a mechanical defect, and therefore, a higher risk of fracture (121617). Furthermore, when growth is rapid, the cortex around a cyst may be destroyed (1), and thus, tumor size alone may not significantly differentiate between benign and malignant tumors.
This study had some limitations. First, the number of cases was relatively small, and the number of malignant tumors was more than twice that of benign tumors, which is probably because malignant bone tumors have a higher associated risk of pathologic fracture. A larger scale study with a similar number of benign and malignant cases is required for more detailed analyses. Second, the various tumor types and their different distributions among benign and malignant cases might have affected our results. Third, more specific studies to distinguish between benign and malignant tumors, such as, positron emission tomography-CT, MR spectroscopy, elastography, dynamic enhancement, and diffusion weighted MRI could not be performed because of the retrospective nature of this study. Fourth, the methods and the equipment used for the imaging studies differed at the institutions involved, although no problems were encountered during the imaging analyses.
In conclusion, the study showed that significant differentiating criteria between benign and malignant pathologic bone fractures included ill-defined tumor margin, the presence of sclerotic tumor margin and an extra-osseous soft tissue mass, the absence of cystic/necrotic/hemorrhagic portions in a mass, the homogeneous enhancement pattern; and furthermore, the presence of a displaced fracture and of underlying cortical change such as permeative or 'moth-eaten' pattern were suggestive of malignant pathologic fractures. Some imaging findings were found to aid in the differentiation between benign and malignant pathologic fractures. Hence, radiologists should be aware of the above mentioned imaging characteristics in making differential diagnoses.
Figures and Tables
Fig. 1
A 35-year-old female patient with an aneurysmal bone cyst of the distal radius. Anteroposterior radiograph (A) showing a well-defined, radiolucent mass lesion with a thin sclerotic rim and endosteal scalloping in the distal radius. Non-displaced, cortical disruption of clear-cut type (arrows) was noted on both sides of the distal radius. No evidence of intra-tumoral mineralization or periosteal reaction was shown. Fat-suppressed, sagittal contrast enhanced image (B) showing thin peripheral rim enhancement with a cystic or hemorrhagic portion within the lesion (arrows).
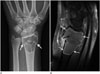
Fig. 2
An 83-year-old male patient with bone metastasis from prostatic cancer of the left femur. Plain radiograph (A) showing an ill-defined osteolytic mass lesion in the diaphysis of the femur. A permeative pattern (arrows) of cortical change was noted. There was no evidence of mineralization. Fat-suppressed, coronal T2-weighted MR image (B) showing extensive soft-tissue edema around the fracture site and an ill-defined bony and soft tissue mass (arrows) in the femoral diaphysis. Fat-suppressed, axial contrast enhanced image (C) showing ill-defined extra-osseous soft tissue formation with relatively homogeneous enhancement (arrows). The bony lesion was also homogeneously enhanced.
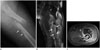
Fig. 3
A 56-year-old female patient with bone metastasis from lung cancer of the right tibia. Reformatted coronal CT scan (A) showing periosteal reaction with a sunburst pattern (arrows) adjacent to the mass lesion. Fat-suppressed, contrast-enhanced coronal T1-weighted MR image (B) showing a heterogeneously enhanced extra-osseous lesion (arrows) as compared with the homogeneous enhancement shown by the intra-osseous portion.
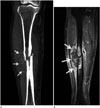
Table 1
Types, Locations, and Numbers of Benign and Malignant Bone Tumors with an Associated Pathologic Fracture

Table 2
Imaging Analysis Results

References
1. Miller SL, Hoffer FA. Malignant and benign bone tumors. Radiol Clin North Am. 2001; 39:673–699.
2. Ortiz EJ, Isler MH, Navia JE, Canosa R. Pathologic fractures in children. Clin Orthop Relat Res. 2005; (432):116–126.
3. Shin DS, Shon OJ, Byun SJ, Choi JH, Chun KA, Cho IH. Differentiation between malignant and benign pathologic fractures with F-18-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography. Skeletal Radiol. 2008; 37:415–421.
4. Springfield DS, Brower TD. Pathologic fractures. Philadelphia: J.B. Lippincott;1984.
5. Bridge JA, Orndal CC. Cytogenetic analysis of bone and joint neoplasms. In : Helliwell T, editor. Pathology of bone and joint neoplasms. Philadelphia: W.B. Saunders;1999. p. 59–78.
6. Springfield DS. Radiolucent Lesions of the Extremities. J Am Acad Orthop Surg. 1994; 2:306–316.
7. Papagelopoulos PJ, Mavrogenis AF, Savvidou OD, Benetos IS, Galanis EC, Soucacos PN. Pathological fractures in primary bone sarcomas. Injury. 2008; 39:395–403.
8. Jackson WF, Theologis TN, Gibbons CL, Mathews S, Kambouroglou G. Early management of pathological fractures in children. Injury. 2007; 38:194–200.
9. Sarahrudi K, Hora K, Heinz T, Millington S, Vécsei V. Treatment results of pathological fractures of the long bones: a retrospective analysis of 88 patients. Int Orthop. 2006; 30:519–524.
10. Coleman RE. Skeletal complications of malignancy. Cancer. 1997; 80:8 Suppl. 1588–1594.
11. Wedin R, Bauer HC, Wersäll P. Failures after operation for skeletal metastatic lesions of long bones. Clin Orthop Relat Res. 1999; (358):128–139.
12. Myers SP. MRI of bone and soft tissue tumors and tumorlike lesions. New York: Thieme;2008.
13. Bui KL, Ilaslan H, Bauer TW, Lietman SA, Joyce MJ, Sundaram M. Cortical scalloping and cortical penetration by small eccentric chondroid lesions in the long tubular bones: not a sign of malignancy? Skeletal Radiol. 2009; 38:791–796.
14. Priolo F, Cerase A. The current role of radiography in the assessment of skeletal tumors and tumor-like lesions. Eur J Radiol. 1998; 27:Suppl 1. S77–S85.
15. Helliwell TR. Pathology of bone and joint neoplasms. Philadelphia: Saunders;1999.
16. Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation. Radiographics. 2001; 21:1283–1309.
17. James SL, Davies AM. Giant-cell tumours of bone of the hand and wrist: a review of imaging findings and differential diagnoses. Eur Radiol. 2005; 15:1855–1866.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download