Abstract
Undifferentiated sarcomas are rare tumors not classified into any sarcoma subtype. Due to their rarity, imaging findings of undifferentiated sarcomas are poorly characterized. The purpose of this report was to present imaging findings of a pathologically confirmed undifferentiated sarcoma originated from the left kidney of a 12-year-old boy. The mass was infiltrative involving the renal pelvis. It mimicked massive hilar lymphadenopathy with a preserved renal contour visible by both ultrasonography and CT. Renal vein thrombosis was also observed. Although undifferentiated sarcomas are rare, they should be considered in differential diagnosis of infiltrative renal masses with renal pelvis invasion in children.
Wilms tumors are by far the most common primary renal tumors in pediatric patients. Other less common malignant primary renal tumors affecting pediatric patients include mesoblastic nephromas, renal cell carcinomas (RCCs), rhabdoid tumors, renal medullary carcinomas, and primary renal sarcomas that are rare (12). Most sarcomas can be categorized into various subtypes according to their ultrastructural morphologies and immunohistochemical staining profiles. However, some sarcomas cannot be classified into a specific subtype even with the most state-of-the-art techniques. These sarcomas are referred to as 'undifferentiated sarcomas' (3). Undifferentiated sarcomas rarely arise from the kidney. Little is known about the imaging findings of these tumors (4). Here we report ultrasonography (US) and computed tomography (CT) findings of a pathologically confirmed undifferentiated sarcoma arising from a kidney in a 12-year-old boy. To the best of our knowledge, this is the first detailed imaging findings of a primary renal undifferentiated sarcoma from a patient in this age group.
The Institutional Review Board at our hospital approved this case study and waived the requirement for informed consent. A 12-year-old boy visited an outpatient clinic due to recurrent left-upper quadrant abdominal pain and vomiting that had persisted for three months. He had no remarkable perinatal or other medical history except a single episode of uncomplicated urinary tract infection in early childhood. On urine analysis, there was minimal hematuria and proteinuria without pyuria. Urine culture was also negative. To determine the cause of abdominal pain, he underwent abdominal US (Fig. 1) which revealed an approximately 5-cm, ill-defined, and heterogeneously hypoechoic lesion occupying the lower pole of the patient's left kidney with a preserved renal contour. The lesion did not appear to exert much mass effect due to its size, suggesting an infiltrative nature. Doppler US revealed minimal vascularity within the mass. Nodular masses at renal hilar area considered as retroperitoneal lymph nodes were also observed in the mid-abdomen.
Due to the long duration of the symptom and negative results from culture, the lesion was considered as tumorous rather than lobar nephronia. An abdomino-pelvic CT scan was performed with contrast enhancement for further evaluation of the mass. CT images (Fig. 2A, B) revealed that the left kidney was enlarged. An ill-defined, generally low-attenuating, and heterogeneously enhancing mass occupied the mid to lower pole of the left kidney. The renal contour was preserved, consistent with US findings. The mass appeared to invade the renal hilum. It was accompanied by thickening and dilatation of the renal pelvis and the proximal ureter. In addition, an obvious contrast filling defect within the left renal vein was visible. It seemed to be a continuation of the mass. This finding suggested malignant renal vein thrombosis. Similar to US findings, multiple nodular lesions suggesting enlarged lymph nodes were noted in the left paraaortic space from the level of the renal hilum to the mid-abdomen. Several small nodules that were deemed to be metastatic nodules were also visible in both lower lung fields of CT images.
Positron emission tomography (PET) was performed using 18F-fluorodeoxyglucose (FDG) (Fig. 2C). Intense FDG uptake was noted not only in the left renal mass, but also in the areas of renal vein thrombosis and hilar masses. No evidence of additional distant metastasis was observed other than small lung nodules detected in the abdomino-pelvic CT images. All these imaging findings from multiple modalities were highly suggestive of a malignant renal tumor. Thus, a US-guided core-needle biopsy was performed.
Pathological examination clearly revealed the malignant nature of the mass. However, histologic type of the tumor could not be specified since all immunohistochemical panels were negative except integrase interactor 1. Therefore, laparoscopic radical left nephrectomy was performed for a definitive diagnosis of the renal mass. During the operation, a severe adhesion around the left kidney accompanied by hilar mass was observed, in accordance with preoperative imaging studies.
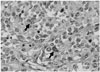
Inspection of gross specimen revealed a diffuse and poorly-bordered whitish mass located in the lower pole of the left kidney. The mass involved the renal papilla. It was 45% necrotic. Light microscopy observations revealed that the mass was a morphologically undifferentiated mesenchymal tumor. It was predominantly composed of small round cells with patternless solid sheet-like growth (Fig. 3). Multiple tumor emboli within renal sinus veins were also observed by microscopy. One notable finding in the pathologic specimen was the formation of tumor nodules in the renal hilar area. These nodules were thought to represent metastatic lymph nodes in pre-operative CT and PET-CT (Fig. 2). However, elaborate observation of the specimen revealed that the nodules were actually formed by direct extensive invasion of the tumor mass beyond the renal sinus into the perirenal space. Multiple immunohistochemical stains were performed for the specimen. Most of these stains yielded negative results, thereby excluding the possibilities of rhabdomyosarcoma, malignant rhabdoid tumor, and sarcomatoid RCC. Ewing sarcoma was also excluded because fluorescent in situ hybridization test for EWSR1 gene translocation was negative. Therefore, the final pathological diagnosis was an undifferentiated sarcoma from the kidney with renal sinus infiltration and tumor thrombosis in the renal vein. The subtype of undifferentiated sarcoma was primitive mesenchymal type. Perinephric fat invasion was also present. After the operation, the patient received adjuvant chemotherapy and whole lung radiotherapy. On follow-up chest and abdomino-pelvic CT at 9-month after surgery, there was no evidence of tumor recurrence at the nephrectomy site. However, progression of lung metastasis was noted with increased number and size of nodules in both lungs.
Various types of malignant primary renal tumors have been documented in children. Imaging findings and clinical presentation can enable the distinction of the type of tumor (15), but not always. Wilms tumors are typically presented as large heterogeneous renal masses with a propensity towards vascular invasion and the displacement of adjacent structures. They tend to occur in patients younger than 5 years of age (1). Occasional bilateral presentation (10%) is another well-known characteristic of Wilms tumors (1). In our case, a Wilms tumor was thought to be unlikely mainly due to the patient's age. The ill-defined margin of the tumor coupled with the massive invasion of the renal sinus and pelvis observed by CT also supported the search for an alternative preoperative diagnosis because it is well known that Wilms tumors rarely occupy the renal pelvis or extend down the ureter (5).
Even though RCC is rare in childhood, it can occur in patients ranging from 10 to 20 years old (1). Usually, RCCs are smaller than Wilms tumors at presentation. They are more frequently presented bilaterally with calcification (25% vs. 9%) and bone metastasis (1). However, invasion of the renal vein or inferior vena cava in pediatric RCC is relatively uncommon (6). By patient age alone, juvenile RCC appeared to be a likely possibility in our case. While juvenile RCC generally distorts the normal renal architecture and forms pseudocapsules (1), the tumor in our case report was infiltrating and replacing the renal parenchyma rather than distorting it.
Renal medullary carcinoma is a highly aggressive tumor that occurs in adolescents and young black adults who exhibit sickle cell traits or hemoglobin sickle cell disease (1). This tumor appears as a centrally located infiltrative lesion invading the renal sinus with peripheral caliectasis, reniform enlargement, and smaller peripheral satellite nodules (1). Mesoblastic nephromas and malignant rhabdoid tumors of the kidney occur in younger patients than Wilms tumors do (1). Renal lymphoma also can occur in children. It can show three patterns: multiple bilateral masses enhancing less than normal renal parenchyma, a focal mass, or a large retroperitoneal mass engulfing the kidney (7). Concomitant retroperitoneal lymph node enlargement is common. Our case showed a focal renal mass with retroperitoneal lymphadenopathy. Although renal lymphoma almost always has other sites of active disease (7), this was not the case in our patient.
Primary renal sarcomas are rare. They occur in less than 1% of all malignant renal tumors (2). Clear cell sarcoma is the major histologic type in children. It appears as a large, well-circumscribed, and solid mass with cystic changes (2). Malignant renal vein thrombosis is less frequent than Wilms tumors (less than 5% vs. 15-20%) (5). However, clear cell sarcoma cannot be differentiated from Wilms tumors by imaging findings alone (1). The imaging characteristics of other childhood renal sarcomas including rhabdomyosarcoma, Ewing sarcoma, and undifferentiated sarcoma are less frequently discussed in medical literatures compared to those of clear cell sarcoma.
An undifferentiated sarcoma of kidney is exceedingly rare in pediatric population. Most of these tumors originate from the extremities or trunk. A review of clinical data from 5746 patients under the age of 21 who were registered in the Intergroup Rhabdomyosarcoma Study group with a diagnosis of rhabdomyosarcoma or undifferentiated sarcoma revealed that only four undifferentiated sarcomas originated from the kidney (8). Most previous reports of undifferentiated sarcomas have focused on their pathologic characteristics. We could find only two articles that described their imaging appearances. Somers et al. (9) reported a case series of 13 patients with undifferentiated sarcoma, of whom 10 were evaluated by CT or magnetic resonance imaging (MRI). This study found that most of the tumors were solid. The majority of them did not seem to be encapsulated. Based on CT and MRI, necrosis, hemorrhage, and calcification were seen in 40%, 20%, and 20% of these tumors, respectively. Stein-Wexler (4) reviewed several cases of soft tissue sarcoma in children and reported that undifferentiated sarcomas showed little enhancement on CT images with attenuation similar to that of muscle. They were heterogeneously hyperintense on T2-weighted MRI images (4). Their enhancement was confined to the periphery of the lesion (4). However, we were unable to find any report describing the imaging appearance of primary renal undifferentiated sarcomas. Our case demonstrated an infiltrative nature accompanied by internal necrosis with little enhancement. Although no hemorrhage or calcification was present, massive renal hilar infiltration mimicking lymphadenopathy, malignant venous thrombosis, and lung metastasis were observed in the initial presentation. Therefore, these manifestations can be clues for the diagnosis of undifferentiated sarcoma.
In conclusion, even though undifferentiated sarcoma is rare, it should still be considered in pediatric patients who exhibit infiltrative and heterogeneous renal mass with renal pelvis invasion and renal vein thrombosis by imaging studies.
Figures and Tables
Fig. 1
Ultrasonography of a 12-year-old boy presented with recurrent abdominal pain and vomiting.
A. Abdominal ultrasonography with longitudinal scan of the left kidney showing an ill-defined and heterogeneously hypoechoic mass (arrows) within the mid to lower pole of the kidney with a preserved renal contour. The mass seems to invade the renal hilum. It is accompanied by pelvic wall thickening (arrowhead).
B. Doppler interrogation of the left kidney revealing some vascularity within the mass with much less vascularity compared to the adjacent normal renal parenchyma.
C. Ultrasonography with transverse scan of the mid-abdomen just below the level of the left renal vein showing hypoechoic nodular lesions (asterisks) considered as enlarged lymph nodes in the left paraaortic area.
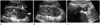
Fig. 2
Further imaging studies of a 12-year-old boy with left renal mass.
A. Abdomino-pelvic computed tomography (CT) of an axial image of the nephrographic phase at the level of the left renal hilum demonstrating an infiltrative renal mass involving the renal pelvis with low-attenuating lesions (arrows) within the expected course of the left renal vein, suggestive
of venous thrombosis.
B. An axial slice of CT slightly caudad to A showing an ill-defined and low attenuating lesion with heterogeneous enhancement replacing the left renal parenchyma with a preserved renal contour. A round low-attenuation lesion (arrow) between the aorta and the left renal tumor is also noted.
This lesion was thought to represent metastatic lymphadenopathy at the time of diagnosis. However, it was later confirmed to be a massive renal hilar and perirenal extension of the tumor.
C. Axial fusion positron emission tomography-CT scan taken on approximately the same level as B showing intense fluorodeoxyglucose uptake in both the left renal and perihilar masses.

References
1. Lowe LH, Isuani BH, Heller RM, Stein SM, Johnson JE, Navarro OM, et al. Pediatric renal masses: Wilms tumor and beyond. Radiographics. 2000; 20:1585–1603.
2. Lalwani N, Prasad SR, Vikram R, Katabathina V, Shanbhogue A, Restrepo C. Pediatric and adult primary sarcomas of the kidney: a cross-sectional imaging review. Acta Radiol. 2011; 52:448–457.
3. Alaggio R, Bisogno G, Rosato A, Ninfo V, Coffin CM. Undifferentiated sarcoma: does it exist? A clinicopathologic study of 7 pediatric cases and review of literature. Hum Pathol. 2009; 40:1600–1610.
4. Stein-Wexler R. Pediatric soft tissue sarcomas. Semin Ultrasound CT MR. 2011; 32:470–488.
5. Siegel MJ, Chung EM. Wilms' tumor and other pediatric renal masses. Magn Reson Imaging Clin N Am. 2008; 16:479–497. vi
6. Downey RT, Dillman JR, Ladino-Torres MF, McHugh JB, Ehrlich PF, Strouse PJ. CT and MRI appearances and radiologic staging of pediatric renal cell carcinoma. Pediatr Radiol. 2012; 42:410–417. quiz 513-514
7. Chepuri NB, Strouse PJ, Yanik GA. CT of renal lymphoma in children. AJR Am J Roentgenol. 2003; 180:429–431.
8. Raney B, Anderson J, Arndt C, Crist W, Maurer H, Qualman S, et al. Primary renal sarcomas in the Intergroup Rhabdomyosarcoma Study Group (IRSG) experience, 1972-2005: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2008; 51:339–343.
9. Somers GR, Gupta AA, Doria AS, Ho M, Pereira C, Shago M, et al. Pediatric undifferentiated sarcoma of the soft tissues: a clinicopathologic study. Pediatr Dev Pathol. 2006; 9:132–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download