INTRODUCTION
Hepatic sinusoidal dilatation (HSD) may be associated with venous outflow impairment such as hepatic sinusoidal obstruction syndrome, Budd-Chiari syndrome, or cardiac disease. In the absence of hepatic venous obstruction, HSD may be related to several other diseases, such as certain systemic inflammatory disorders, granulomatous and neoplastic diseases, and hematological malignancies (12). It may also be related to systemic chemotherapy for malignancy such as colorectal cancer, pregnancy, and oral contraceptive use (345).
Here, we describe ultrasonography (US), computed tomography (CT), and magnetic resonance imaging (MRI) findings of the HSD in our case. We report the useful MRI finding of HSD that it has connection to the hepatic or portal vein, reliably identified on the hepatobiliary phase (HBP).
CASE REPORT
A 37-year-old woman was referred to our institution from a local medical clinic due to an incidentally-detected large hepatic mass on US. The patient had no complaints of symptoms or past medical history. A physical examination and laboratory findings showed no abnormality.
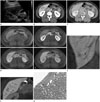
On transabdominal US examination in our hospital, a 3-cm sized, non-specific hypoechoic mass in hepatic segment VI relative to the surrounding liver was detected (Fig. 1A). The focal hepatic lesion was not defined on the non-enhanced CT images. On the contrast enhanced dynamic CT scan of the liver, this focal hepatic lesion showed delayed enhancement (Fig. 1B, C).
Liver MRI was then performed with dynamic imaging, including imaging in the arterial phase (30 sec), portal venous ph-ase (60 sec), and transitional phase (3 min), as well as the HBP (10 min), after administration of 0.1 mmol/kg Gd-EOB-DTPA (Primovist; Bayer Healthcare, Berlin, Germany). On T1-weighted images, the focal hepatic lesion was slightly hypointense (Fig. 1D). On T2-weighted half-Fourier acquisition turbo spin echo (HASTE) images, it was isointense to the adjacent liver parenchyma. In comparison with the CT scans, a similar enhancement pattern was observed on the enhanced dynamic MR images (Fig. 1D). An ill-defined, vessel-like hypointense structure was best depicted, especially on the HBP than on any other phase. In addition, connection with the peripheral branches of both the right portal vein and middle hepatic vein was shown on the HBP (Fig. 1E, F). In the diffusion-weighted image and apparent diffusion coefficient map, no definite diffusion restriction was seen.
A sonographic-guided needle biopsy was performed, and a microscopic examination showed only HSD without other histological abnormalities (Fig. 1G).
DISCUSSION
HSD can occur in various conditions. If HSD is the only abnormal finding in the liver, other systemic diseases may coexist. Therefore, further medical investigation for neoplastic or granulomatous disease elsewhere in the body is needed (5).
To our knowledge, imaging findings of localized HSD in a patient without any other medical disorders or oral contraceptive therapy have not been described in the literature.
Weinberger et al. (6), described sonographic findings of a hyperechoic hepatic mass. In comparison, in our case, HSD was shown as a hypoechoic mass in the liver on US, equivalent to the other previous case (7).
Yang et al. (7), described the crossing vascular structures within the slightly hyperintense lesion on T2-weighted HASTE images as an interesting MRI finding. However, in our case, since the lesion was not shown on T2-weighted HASTE images, it was not possible to evaluate the presence of the crossing vascular structures. On the HBP images, we found that HSD had a connection to both the middle hepatic vein and the right portal vein. This useful imaging finding is highly suggestive of vascular lesions, because the hepatic sinusoidal space is anatomically connected to the hepatic vessels. In addition, the reticular vessel-like lesion was shown with no mass effect or occlusion. This helps to distinguish HSD from hepatic neoplasms, because the extension of normal hepatic vessels to large hepatic neoplasms is extremely rare, with the exception of a few malignancies in the liver (8). Shin et al. (9), reported that reticular hypointensity on Gd-EOB-DTPA MR HBP images is highly specific for the diagnosis of sinusoidal obstruction syndrome in patients with treated co-lorectal hepatic metastases. Comparing their study with our case, there are some differences; the connection of HSD with hepatic vessels was not identified, and chemotherapy for colorectal hepatic metastases induced HSD.
Histologically, focal HSD is associated with hepatocyte atrophy and necrosis.
Therefore, we believe that reticular hypointensity on MR HBP images is attributed to the absence or insufficiency of functional hepatocytes within the lesion, which is needed for contrast uptake. Undamaged normal hepatic tissues in the center and periphery of HSD may also contribute to the reticular shape and poorly defined margin of these lesions.
Peliosis hepatis should be included in the differential diagnosis. It is defined as cystic dilatation of the hepatic sinusoidal space filled with red blood cells on microscopic examination. To distinguish peliosis hepatis from sinusoidal dilatation, hepatic lesions should have evidence of disruption of reticulin fibers supporting the hepatocytes and sinusoids. In peliosis hepatis, the radiological findings are variable depending on the size and stage of the hemorrhage. Small (< 2 cm) peliosis hepatis may be undetectable on imaging studies, however, large peliosis hepatis typically show a sign of progressive centrifugal enhancement on the portal and equilibrium phases without mass effect on adjacent hepatic vessels (10).
In conclusion, detecting a connection between the focal hepatic lesion and portal or hepatic veins on the delayed HBP MR images could be one of the diagnostic imaging clues for HSD. However, a potential limitation for the present study is that the connection of HSD with hepatic vessels was not pathologically confirmed due to an insufficient specimen.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download