Abstract
Purpose
The purpose of this study was to analyze the incidence and predictive factors of tandem cervical spinal stenosis (CSS) in patients with lumbar spinal stenosis (LSS) based on MR.
Materials and Methods
From January to May of 2011, 140 consecutive patients (36 men, 94 women; mean age, 68.9 years; age range, 60-87 years) were included for the analysis. All patients were at least 60 years old, had undergone lumbar spine MRI including additional cervicothoracic sagittal T2-weighted images, and were clinically suspected of LSS. Two spine radiologists evaluated the risk factors for CSS, considering the possible risk factors such as age, sex, alignment disorder of the lumbar spine, number of levels of LSS, and severity of LSS, based on MR.
Results
Of the 140 patients, 42 (30%) patients had tandem spinal stenosis. CSS was more common among patients with LSS (42 of 61, 69%) than among patients without LSS (27 of 79, 34%) (p = 0.000). Grade 2 or 3 CSS was more commonly observed among patients with grade 2 or 3 LSS (15 of 53, 28%), than among patients with grade 0 or 1 LSS (8 of 87, 9%) (p = 0.003). Only the severity of LSS showed a significant association with the severity of CSS (p = 0.045).
Degenerative spinal stenosis in the lumbar or cervical spine is a common disease in the elderly population, because these are the most mobile segments of the spine. Degenerative spinal stenosis can present as concurrent lumbar spinal stenosis (LSS) and cervical spinal stenosis (CSS), which was first described by Teng and Papatheodorou (1) in 1964. Several studies-performed using clinical or radiologic diagnoses based on radiography, myelography, or CT findings only in symptomatic patients-have shown the incidence of tandem spinal stenosis (TSS) to range from 5% to 25% (234). The incidence of TSS in the asymptomatic population is expected to be higher than suggested in past reports.
MR imaging is now widely used for the evaluation and diagnosis of spinal disorders (5). For the past several years in our institute, additional cervicothoracic sagittal T2-weighted images (CT-sag-T2WI) have been routinely obtained in the lumbar spine MR images for all patients. Using spine coils for scanning the lumbar spine, additional CT-sag-T2WI can easily be obtained without changing the coils and with an additional scan time of less than two minutes. We have frequently observed TSS in the cervical and lumbar spine on MRI. Nevertheless, there are few reports of stenosis in the cervical and lumbar spine on MRI. Invasive treatment, such as surgery, is mandatory for severe or continuous cases of degenerative lumbar stenosis. However, when CSS is also present, improper positioning during the operation may aggravate symptoms (6). Therefore, patients should be ch-ecked for accompanying CSS when planning for the treatment.
Our hypotheses were as follows: 1) patients showing LSS would have CSS more frequently; and 2) patients with more severe LSS would tend to have more severe CSS, because both genetic predisposition and environment could affect the entire spinal column. The aim of our study was to evaluate the incidence and predictive factors of tandem CSS in patients with LSS, based on MR imaging findings.
This retrospective study was approved by our Institutional Review Board. Informed consents were waived. The spinal stenosis is commonly seen with aging. Therefore, patients over 60 years of age were enrolled in this study, who had lumbar spine MR studies including additional CT-sag-T2WI at our institute during the period between January and May of 2011. Exclusion criteria were as follows: 1) under 60 years of age; 2) an MR indication of recent trauma, neoplasm, or infection; and 3) a previous spine operation.
From January to May of 2011, lumbar spine MR studies were performed in 415 patients at our institute. Among them, 210 patients were over 60 years old. Of these 210 patients, 70 patients were excluded for the following reasons: compromised lumbar central canal due to recent trauma (n = 17), previous lumbar or cervical surgical operation (n = 41), neoplasm/intradural extramedullary tumors (n = 5), metastasis (n = 2), and infection (n = 5). The final study group was comprised of 140 patients (age range, 60-87 years; mean age, 69.3 years). There were 94 women (age range, 60-86 years; mean age, 68.8 years) and 36 men (age range, 60-87 years; mean age, 70.0 years).
All MRI examinations were obtained with two 3.0-T scanners (Achieva, Philips Medical Systems, Best, the Netherlands) and two 1.5-T scanners (Gyroscan Intera, Philips Medical Systems, Best, the Netherlands; Intera, Philips Medical Systems, Best, the Netherlands), using a five-channel synergy spine coil. Sagittal T1WI and T2WI and axial T1WI and T2WI were used for conventional lumbar spine MR imaging [repetition time (TR)/echo time (TE), 500/15 for T1WI and 3600/120 for T2WI; slice thickness, 4 mm; slice gap, 0.4 mm; field of view, 32 cm for sagittal images and 16 cm for axial images; matrix, 512 × 512; flip angle, 90°; and excitations, 3].
In addition to the conventional MRI sequences, a cervicothoracic sagittal T2-weighted spin-echo sequence (TR/TE, 3000-4000/100; number of signals acquired, 2; matrix size, 512 × 512; slice thickness, 4 mm; acquisition time, 1 minute 40 seconds) was obtained for all MR lumbar imaging examinations. This sequence was obtained by using the same five-channel synergy spine coil.
Two spine radiologists, with ten years and one year of experience in spine MR interpretation, reviewed the MR images in consensus. For all subjects, the following were evaluated: severity and presence of LSS and CSS, alignment disorder of the lumbar spine such as degenerative spondylolisthesis or retrolisthesis, and number of levels of LSS.
Spinal canal stenosis was defined as narrowing of the spinal canal resulting from spondylosis, disc bulging, hypertrophy of ligamentum flavum, or ossification of ligamentum flavum. LSS was divided into four grades, according to the degree of separation of the cauda equina on axial T2WI. Grade 0 was defined as no LSS, as the anterior cerebrospinal fluid (CSF) space was not obliterated. Grade 1 was defined as mild LSS, in which the anterior CSF space was mildly obliterated but all cauda equina could be clearly separated from each other. Grade 2 was defined as moderate LSS, in which the anterior CSF space was moderately obliterated and some of the cauda equina were aggregated, making it impossible to visually separate them. Finally, grade 3 was defined as severe LSS, in which the anterior CSF space was obliterated severely as to show marked compression of the dural sac, and none of the cauda equina could be visually separated from each other but appeared as one bundle (Fig. 1) (7).
Under the approval of the spine specialist of our institution, CSS was divided into four grades according to the extent of CSF space stenosis revealed from T2WI. The extent was calculated from the degree (D) of CSF reserve at the site of stenosis, using the formula mentioned below. The followings were measured: anteroposterior dimension of the non-stenotic central canal (A), that of the spinal cord (B), and that of the stenotic central canal (C) (Fig. 2).
The four categories based on the degree of CSF reserve were as follows: grade 0 (D > 50%), grade 1 (5% < D ≤ 50%), grade 2 (-5% < D ≤ 5%), and grade 3 (D ≤ -5%). The CSS grading system was based on a consensus between multiple specialists involved in specialized spine care within the institution.
The patients were divided into two groups, depending on the presence or absence of spinal stenosis. Next, the patients were further divided into two more groups: absent or insignificant spinal stenosis (grade 0 and 1) and significant spinal stenosis (grade 2 and 3). The chi-square test was done to evaluate the two groups.
We investigated the relations regarding hypothesized predictive factors of CSS including age, sex, alignment disorder of the lumbar spine, number of levels of LSS, and severity of LSS. The age difference between the absent or insignificant spinal stenosis group and the significant spinal stenosis group was evaluated using the Mann-Whitney U test. There were three groups based on age: between 60 and 69 years of age, between 70 and 79 years of age, and over 80 years of age. Regarding the number of levels of LSS, one group had either no stenosis or only a single level, while the other group had more than two levels. A univariate analysis of risk factors was performed for the second grouping regarding CSS (absent or insignificant spinal stenosis, significant spinal stenosis). Fisher's exact test was performed for age, while the chi-square test was performed for all other risk factors. Furthermore, a logistic regression analysis was carried out for all lumbar spine MRI findings that were regarded as risk factors of significant CSS. A p-value of 0.05 was considered to indicate a statistically significant difference. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows version 17.0 (SPSS Inc., Chicago, IL, USA).
MR imaging revealed that 61 patients (40.6%) had LSS. Both CSS and LSS were seen in 42 patients (30.0%). CSS was more common among patients with LSS (42 of 61, 68.9%) than among those without LSS (27 of 79, 34.2%) (odds ratio = 16.911, p = 0.000) (Table 1). Grade 2 or 3 CSS was more commonly observed among patients with grade 2 or 3 LSS (15 of 53, 28.3%), than in patients with grade 0 or 1 LSS (8 of 87, 9.2%) (p = 0.003) (Table 2).
Table 3 summarizes the univariate analysis of lumbar spine MRI findings and characteristics of the patients, which are regarded as risk factors of CSS. Significant CSS was more apparent in the cases with multiple segments of LSS and in the cases with significant LSS (p = 0.007, 0.003, respectively). The logistic regression analysis revealed that severity of LSS was the only significant predictive factor for CSS (odds ratio = 3.547, p = 0.045) (Fig. 3).
Presence of lumbar alignment disorder, number of levels of LSS, age, and sex were not significantly different between patients with and without CSS (p = 0.451, 0.505, 0.633, 0.272, respectively).
TSS refers to the occurrence of concurrent LSS and CSS. Dagi et al. (2) were the first to use the term "TSS" in 1987, to describe the combined stenosis. There are various treatment approaches. For instance, some suggest sequential surgery starting with the most symptomatic area, while others recommend treatment of the cervical spine first (489). Still others suggest surgical treatment of both simultaneously (11011). Regardless of the approach, it is crucial that the involvement of both regions is recognized. Symptoms of CSS may be mistaken for those of LSS, leading to erroneous treatment decisions or repeated invasive treatments. Furthermore, missed cervical spinal conditions may lead to improper positioning during operations or nerve injuries during position changes (6).
By providing high-resolution images, MR imaging plays an important role in the detection and diagnosis of spinal stenosis. In Thomé's study, LSS was found on MRI in more than 20% of the cases of patients over 60 years of age (12). In our study, LSS cases were found in 43.6% of the patients over 60 years of age (a higher percentage than suggested in previous reports), perhaps because the MR was scanned in symptomatic patients.
In previous studies, the incidence of TSS has been estimated to range from 5% to 25% (234). These statistics are mostly based on clinical findings, radiography, myelography, and CT. Very few studies have used MR imaging to investigate the relationship.
Lee et al. (13) reported the incidence of asymptomatic cervical cord compression in LSS patients at 23.7%, based on MR studies. Unlike in out study, this study included patients with LSS. In our study, cervical cord compression was observed in 14 out of 140 patients (10%) with symptoms suggestive of LSS (e.g., lumbar back pain, lumbar radicular pain, and neurogenic claudication) and in 9 out of 61 patients (14.8%) with LSS discovered by MRI. In addition, male patients and those with multiple-segment LSS were more likely to have CSS, than female patients and those with single-segment LSS. The diagnosis of LSS and CSS was based on sagittal T2WI only. However, our study included patients with symptoms suggestive of LSS (e.g., lower back pain, lumbar radicular pain, neurogenic claudication, and/or sciatica). The incidence of TSS was 30.0% (42 of 140). Presence of a lumbar alignment disorder, number of levels of LSS, age, and sex were not associated with statistically significant differences. In our study, most LSS diagnoses were made by reviewing both cross-sectional images and sagittal images, as cross-sectional images lead to more accurate LSS diagnoses than sagittal images (14).
In our study, subjects were divided using two different methods. First method was based on the presence or absence of LSS, and the second method was based on whether the patients suffered from no/insignificant spinal stenosis or significant spinal stenosis. The two grouping methods were shown to be statistically significantly different from each another. Patients with significant LSS may have significant CSS. The main indications for surgery are the failure of non-operative management to relieve the patient's symptoms over several months and symptoms that are significant enough to interfere with the patient's quality of life. Grade 2 to 3 central canal stenosis had a closer relationship with aggressive treatment including surgical intervention. Therefore, additional cervical spinal imaging is useful in patients with more severe LSS. However, 34.2% of the patients without LSS also had CSS (Fig. 4). Therefore, routine CT-sag-T2WI is meaningful in both patients with and without LSS. Our lumbar spine MR protocol uses a five-channel spine coil, which allows access to the cervical spine without changing the patient's position. This protocol, which includes CT-sag-T2WI, added 1 minute and 40 seconds to the acquisition time. We strongly suggest CT-sag-T2WI be included in routine lumbar spine MR protocols.
This study had several limitations. First, it was a retrospective study based on radiologic reports only. The LSS and CSS grading systems used were not compared to patients' symptoms or clinical outcomes. Currently, the known grading systems for spinal stenosis have certain problems. For LSS, the method of calculating dural sac cross-sectional area and dural sac anterior to posterior dimension has been used, but it is time-consuming and does not consider the nerve roots inside of the dural sac. Compared to calculating the cross-sectional area, the grade tends to be lower in the lower lumbar areas (1415). However, it is more intuitive to use and easier to grade than the previous method (1516). It is also simpler to communicate between surgeons and radiologists.
CSS has been graded according to the extent of CSF space loss, which tends to give readings with lower grades of spinal stenosis, compared to the clinical symptoms. This is owing to the non-loss of signal intensity from CSF, even if it is actually deformed by the stenosis (16). Thus, it seems reasonable and more accurate to compare the extent of CSF space loss by measuring the diameter, not right at the level of stenosis but rather just above or below the level of stenosis. In this study, diameters at the level of spinal canal stenosis, diameters at the level without stenosis, and diameters of the spinal cord were measured individually. Further studies are necessary to determine if similar results are demonstrated when they are compared directly without using the tools in Picture Archiving and Communication System. As for the third limitation, sagittal T2WI were taken within 1 minute and 40 seconds, without changing the patient's position, with the aid of a spine coil. Without the spine coil, however, changing the patient's position can be inconvenient. Fourthly, there was even a case in this study where the operation was performed because of cervical myelopathy. However, cervical myelopathy may be an etiology of failed back surgery syndrome (17). Finally, the incidence of TSS in our study was 30.0% (42 of 140), which is slightly higher than previously reported in the literature. This is likely to be related to differences in the patient population. The study was conducted in a tertiary hospital, with a large referral base for spine procedures. In addition, the study subjects were selected from patients with symptoms suggestive of LSS, and the incidence of TSS in asymptomatic patients may be higher than that in symptomatic patients.
In conclusion, this study revealed a high frequency of concurrent LSS and CSS and two risk factors of significant CSS (severity of LSS and number of levels of LSS), with the aid of MRI. Thus, it is recommended that all patients with LSS to undergo MRI investigation, to be checked for CSS as well.
Figures and Tables
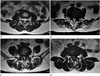 | Fig. 1Grading system of LSS using axial T2-weighted MR images.
A. Grade 0, in which the anterior CSF space was not obliterated.
B. Grade 1, in which the anterior CSF space was mildly obliterated but all cauda equina could be clearly separated from each other.
C. Grade 2, in which the anterior CSF space was moderately obliterated and some of the cauda equina were aggregated, making it impossible to visually separate them.
D. Grade 3, in which the anterior CSF space was obliterated severely as to show marked compression of the dural sac and none of the cauda equina could be visually separated from each other, appearing instead as one bundle.
CSF = cerebrospinal fluid, LSS = lumbar spinal stenosis
|
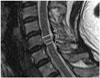 | Fig. 2Grading system of CSS using sagittal T2-weighted images. Grade 0, in which D is greater than 50%. Grade 1, in which D is less than or equal to 50% and greater than 5%. Grade 2, in which D is less than or equal to 5% and greater than -5%. Grade 3, in which D is less than or equal to -5%.D = (C - B) / (A - B) × 100 (%)
A: AP dimension of the non-stenotic central canal, B: AP dimension of the spinal cord, C: AP dimension of the stenotic central canal, D: Fraction of CSF reserve.
AP = anteroposterior, CSF = cerebrospinal fluid, CSS = cervical spinal stenosis, LSS = lumbar spinal stenosis
|
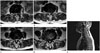 | Fig. 3An 83-year-old woman with claudication.
A-D. Axial T2-weighted MR images show grade 1 central canal stenosis at L1-2 and L2-3 and grade 2 central canal stenosis at L3-4 and L4-5.
E. The sagittal T2-weighted MR image demonstrates grade 2 central canal stenosis at C4-5 and C5-6 (arrows) and grade 1 central canal stenosis at C6-7 (arrowhead). Ossifications of ligamentum flavum are present at T8-9 and T9-10.
|
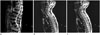 | Fig. 4A 73-year-old man with lower back pain.
A. The sagittal T2-weighted MR image shows L5-S1 degenerative spondylolisthesis. There is no spinal stenosis at the lumbar spine.
B, C. The sagittal T2-weighted MR image shows grade 2 central canal stenosis at C4-5 (arrow) and grade 1 central canal stenosis at C2-3 and C5-6 (arrowheads). A block vertebra is present at C3-4.
|
Table 1
Comparison According to Existence of LSS and CSS: Absence versus Presence

| Patients without CSS (n = 71) | Patients with CSS (n = 69) | p-Value | |
|---|---|---|---|
| Patients without LSS | 52 (65.8) | 27 (34.2) | 0.000 |
| Patients with LSS | 19 (31.1) | 42 (68.9) |
Table 2
Comparison According to Severity of LSS and CSS: Absent or Insignificant Spinal Stenosis versus Significant Spinal Stenosis

| Absent or Insignificant CSS (n = 117) | Significant CSS (n = 23) | p-Value | |
|---|---|---|---|
| Absent or insignificant LSS | 79 (90.8) | 8 (9.2) | 0.003 |
| Significant LSS | 38 (71.7) | 15 (28.3) |
Table 3
Univariate Analysis of Possible Risk Factors for CSS

Absent or insignificant spinal stenosis = grade 0 or 1, significant spinal stenosis = grade 2 or 3. Values inside parentheses indicate percentages, except for age. Values inside parentheses for age indicate standard deviation.
*Mann-Whitney U test.
†Fisher's exact test.
CSS = cervical spinal stenosis, LSS = lumbar spinal stenosis
References
1. Teng P, Papatheodorou C. Combined cervical and lumbar spondylosis. Arch Neurol. 1964; 10:298–307.
2. Dagi TF, Tarkington MA, Leech JJ. Tandem lumbar and cervical spinal stenosis. Natural history, prognostic indices, and results after surgical decompression. J Neurosurg. 1987; 66:842–884.
3. LaBan MM, Green ML. Concurrent (tandem) cervical and lumbar spinal stenosis: a 10-yr review of 54 hospitalized patients. Am J Phys Med Rehabil. 2004; 83:187–190.
4. Epstein NE, Epstein JA, Carras R, Murthy VS, Hyman RA. Coexisting cervical and lumbar spinal stenosis: diagnosis and management. Neurosurgery. 1984; 15:489–496.
5. Aydogan M, Ozturk C, Mirzanli C, Karatoprak O, Tezer M, Hamzaoglu A. Treatment approach in tandem (concurrent) cervical and lumbar spinal stenosis. Acta Orthop Belg. 2007; 73:234–237.
6. Harrison DE, Cailliet R, Harrison DD, Troyanovich SJ, Harrison SO. A review of biomechanics of the central nervous system--part II: spinal cord strains from postural loads. J Manipulative Physiol Ther. 1999; 22:322–332.
7. Lee GY, Lee JW, Choi HS, Oh KJ, Kang HS. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011; 40:1033–1039.
8. Hsieh CH, Huang TJ, Hsu RW. Tandem spinal stenosis: clinical diagnosis and surgical treatment. Changgeng Yi Xue Za Zhi. 1998; 21:429–435.
9. Naderi S, Mertol T. Simultaneous cervical and lumbar surgery for combined symptomatic cervical and lumbar spinal stenoses. J Spinal Disord Tech. 2002; 15:229–231. discussion 231-232
10. Kikuike K, Miyamoto K, Hosoe H, Shimizu K. One-staged combined cervical and lumbar decompression for patients with tandem spinal stenosis on cervical and lumbar spine: analyses of clinical outcomes with minimum 3 years follow-up. J Spinal Disord Tech. 2009; 22:593–601.
11. Eskander MS, Aubin ME, Drew JM, Eskander JP, Balsis SM, Eck J, et al. Is there a difference between simultaneous or staged decompressions for combined cervical and lumbar stenosis? J Spinal Disord Tech. 2011; 24:409–413.
12. Thomé C, Börm W, Meyer F. Degenerative lumbar spinal stenosis: current strategies in diagnosis and treatment. Dtsch Arztebl Int. 2008; 105:373–379.
13. Lee SH, Kim KT, Suk KS, Lee JH, Shin JH, So DH, et al. Asymptomatic cervical cord compression in lumbar spinal stenosis patients: a whole spine magnetic resonance imaging study. Spine (Phila Pa 1976). 2010; 35:2057–2206.
14. Lee MJ, Garcia R, Cassinelli EH, Furey C, Riew KD. Tandem stenosis: a cadaveric study in osseous morphology. Spine J. 2008; 8:1003–1006.
15. Sirvanci M, Bhatia M, Ganiyusufoglu KA, Duran C, Tezer M, Ozturk C, et al. Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J. 2008; 17:679–685.
16. Muhle C, Metzner J, Weinert D, Falliner A, Brinkmann G, Mehdorn MH, et al. Classification system based on kinematic MR imaging in cervical spondylitic myelopathy. AJNR Am J Neuroradiol. 1998; 19:1763–1771.
17. Slipman CW, Shin CH, Patel RK, Isaac Z, Huston CW, Lipetz JS, et al. Etiologies of failed back surgery syndrome. Pain Med. 2002; 3:200–214. discussion 214-217




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download