Abstract
Purpose
To evaluate the effectiveness of fluoroscopy-guided intra-articular facet joint steroid injection for the management of low back pain, and to document the incidence of epidural leakage.
Materials and Methods
In total, 320 facet joint injections of 244 consecutive patients were included in this study. All patients had undergone an intra-articular facet joint steroid injection in 2007 and had follow-up post-treatment medical records. The response to treatment was analyzed on the basis of chart documentation (aggravated, no change, slightly improved, much improved, no pain). Fluoroscopic arthrograms of the injections were retrospectively analyzed by two radiologists.
Results
Of the 244 patients, 85.2% (n = 208) showed improvement after an initial intra-articular facet joint steroid injection. A total of 77.9% (n = 162) of the patients showed symptom recurrence, with a median of a 69 day symptom-free interval, while 30.3% (n = 74) of the patients showed symptom-free intervals of more than six months. Overall, 74 (33.3%) of the 222 cases of intra-articular facet joint steroid injections without concomitant epidural steroid injection showed epidural leakage in fluoroscopic arthrograms.
Facet joint pain is defined in a functional capacity as pain originating from a facet joint, and is a common cause of low back pain, with a prevalence rate ranging from 15% to 52% (1234). Clinical symptoms and radiological findings related to facet joint syndrome are unreliable for the diagnosis of facet joint pain, so that the diagnosis "facet joint syndrome" is made clinically and by excluding other causes of low back pain (56).
Facet joint pain can be managed by nonsurgical interventions, including intra-articular facet joint steroid injections, medial branch blocks, neurolysis of medial branch nerves, and radiofrequency denervation of medial branch nerves. Of these interventions, intra-articular facet joint steroid injection is helpful for the diagnosis (78) and management (91011) of facet joint syndrome. The use of intra-articular steroid injections is currently increasing (12). However, its usage is controversial with respect to the management of chronic low back pain. Some systematic reviews have concluded that there is only limited evidence that intra-articular facet joint steroids can be used to manage this type of pain (71314); other reviews have suggested that there is moderate evidence of its success as a therapy (115). Several studies have reported that intra-articular facet joint injection was effective in short-term follow-up, but ineffective overall in long-term follow-up, even when administered under fluoroscopic guidance (161718).
To the best of our knowledge, no reports have yet documented the long-term results of fluoroscopically-guided intra-articular facet joint steroid injection over more than two years for large numbers of patients. Moreover, no report has yet analyzed the fluoroscopic arthrograms pattern. Although several reports have analyzed outcome predictors, these studies looked at the correlation between the efficacy of intra-articular facet joint steroid injection and clinical findings in only small numbers of patients (1920). Similar studies to investigate outcome predictors, including fluoroscopic arthrographic findings, have not yet been performed on a large numbers of patients.
The purpose of this study was therefore to evaluate, by a retrospective review of medical records and fluoroscopic arthrograms, the success rate and effectiveness of fluoroscopy-guided intra-articular facet joint steroid injection for the management of low back pain. In addition, fluoroscopic arthrogram patterns and the effectiveness of the facet joint injection were also analyzed with a focus on epidural leakage.
Institutional Review Board approval was obtained, and informed consent was not required, for the retrospective review of medical records. All patients who had undergone at least one intra-articular facet joint steroid injection in 2007 were identified using a computerized database at our hospital. A total of 364 intra-articular facet joint steroid injections were performed in 281 patients in our department in 2007. The diagnosis of facet joint syndrome was based on the clinical presentation. An intra-articular facet joint steroid injection was considered for patients presenting with the following: 1) severe low back pain [verbal numeric rating score (0-10) > 5.0]; 2) low back pain that was aggravated by position change including forward flexion and extension rotation; 3) focal tenderness in the paravertebral area in the lower lumbar level; and 4) low back pain without response after more than one month of conservative treatment, such as medication and physical therapy or persistent low back pain after a previous epidural steroid injection (21).
The target site in the facet joints was selected on the basis of fluoroscopic manual compression. Under fluoroscopy, thumb pressure of the patient's lower back over the facet joint determined the tender point for the target facet joint. If the facet joint was unclear on fluoroscopic manual compression, facet joint levels near the area where the patient complained of pain were determined for injection. In the case of a postoperative facet joint injection, the injection was performed at the facet joint level, where the joint space was visualized near the focal tenderness area.
The inclusion criteria were as follows: 1) intra-articular facet joint steroid injection for low back pain, 2) fluoroscopic guidance for intra-articular injection, and 3) the presence of follow-up medical records after the injection. The exclusion criteria were as follows: 1) absence of follow-up data (n = 35 cases); 2) diagnostic facet joint block (n = 5 cases); or 3) no fluoroscopic images (n = 4 cases). A total of 320 intra-articular facet joint steroid injections in 244 patients met these criteria and were included in this investigation.
One of two spine radiologists (one with one year and the other with six years of experience) from our institute performed all intra-articular facet joint steroid injections under fluoroscopic guidance. The patients lay prone on the fluoroscopy table. A uniplanar digital subtraction angiography unit (Integris Allura Xper FD 20; Philips, Best, The Netherlands) was used for fluoroscopy. Following sterile skin preparation and after a fenestrated sterile drape was placed, fluoroscopy was used to check the level and anatomy of the facet joint. Typically, a lateral to medial angulation of between 30° and 40° was necessary to visualize the facet joint space. In cases of L5-S1 facet joint injection, additional craniocaudal angulation was used to avoid conflict between the needle tract and the iliac crest. A 22 or 25-G spinal needle was directed towards the inferior recess of the facet joint coaxial to the X-ray beam using a posterolateral approach under the oblique view of the fluoroscopy. After the needle had located the inferior recess of the facet joint, a minimal quantity of contrast agent [Omnipaque 300 (iohexol, 300 mg iodine per milliliter); Amersham Health, Princeton, NJ, USA] was injected under fluoroscopy. The intra-articular space was identified by examination of the contrast intra-articular sigmoid linear filling pattern (Fig. 1). Subsequently, 20 mg (0.5 mL) of triamcinolone acetonide suspension (Tamceton 40 mg per mL; Hanall Pharmaceutical, Seoul, Korea) and 0.5 mL bupivacaine hydrochloride (0.5 mL/0.5%; Marcaine Spinal 0.5% Heavy; AstraZeneca, Westborough, MA, USA) were injected into the intra-articular facet joint.
Follow-up after the intra-articular facet joint steroid injection was routinely scheduled for 2-4 weeks, according to the patient's condition. We told patients that they could postpone the scheduled follow-up if their symptoms were still tolerable and return to our hospital when the symptoms recurred. At follow-up, the outcome was measured on a 5-point patient satisfaction scale (no pain = virtually pain free; much improved = satisfactory effect; slightly improved = some effect but unsatisfactory; no change = ineffective; aggravated = pain provocation) and was recorded on the medical chart. The next follow-up was usually scheduled 2 or 3 months later. In accordance with the guidelines of the American Society of Interventional Pain Physicians, any kind of steroid injection was performed a maximum of six times per year in our department (2223).
The items reviewed in the medical records of the patients included the age, gender, previous operation history, symptoms, date of facet joint injection, date of the first follow-up and response after facet joint injections, the presence of recurrence, symptoms controllable at last follow-up date or revisit date due to symptom recurrence, duration of symptom relief, and total number of facet joint injections. The retrospective review of the patients' medical records was conducted by one spine radiologist in January 2010.
The symptoms were categorized as low back pain only or low back pain with leg pain. The response after an intra-articular facet joint steroid injection was based on chart documentation and determined by the 5-point patient satisfaction scale. Management after the first intra-articular facet joint steroid injection was classified as follows: observation, repeat intra-articular facet joint steroid injection, other interventions including epidural steroid injection, and others such as operation, medication, or physical therapy. If there was any symptom recurrence, it was recorded as "revisit date due to symptom recurrence". For the patients whose symptoms did not recur, the last follow-up date was recorded as "symptoms controllable at last follow-up date" for the statistical analysis of the symptom-free interval. Those patients who showed improvement after an intra-articular facet joint steroid injection, but who later had symptom recurrence, were grouped as follows: less than 30 days; 31-60 days; 61-180 days; 181-365 days; or more than 366 days.
The items reviewed in the fluoroscopic images included facet joint injection level, success of intra-articular facet joint injection, and the presence of epidural leakage after facet joint injections. Fluoroscopic arthrograms of intra-articular facet joint steroid injections were retrospectively analyzed by two radiologists in consensus.
The success of intra-articular facet joint steroid injections was grouped as follows: failure (peri-articular injection of all targeted facet joints); partial success (one or more peri-articular injection of all targeted facet joints); and complete success (intra-articular injection of all targeted facet joints).
The fluoroscopic images of facet joint injections were checked, focusing on the presence of epidural leakage (Fig. 2) after contrast injection by two radiologists in consensus. Concomitant epidural steroid injection cases (n = 98) were excluded from this analysis; in total, 222 facet joint injections were analyzed for the presence of epidural contrast leakage after an intra-articular facet joint injection.
Outcome data were analyzed with SPSS software (SPSS, version 15; SPSS Inc., Chicago, IL, USA). To determine the median symptom-free interval after improvement from an intra-articular facet joint injection, the Kaplan-Meier method was used. "Symptoms controllable at last follow-up date" or "revisit date due to symptom recurrence" were used for the statistical analysis of the symptom-free interval. Instead of the recurrence date, we used the date of the revisit to our hospital due to symptom recurrence because the onset of the symptom recurrence was vague in most patients with chronic low back pain.
To evaluate the outcome predictors after an initial facet joint injection and the effect of epidural leakage of intra-articular facet joint injections, the responses to facet joint injection were classified as follows: positive (including no pain, much improved, and slightly improved) or negative (including no change and aggravated). Also, to evaluate the effect of epidural leakage of intra-articular facet joint injections, the duration of symptom relief after facet joint injections were classified as more than 2 months or less than 2 months. Fisher's exact test was used for these analyses and a value of p < 0.05 was considered statistically significant.
In total, retrospective data from 320 facet joint injections of 244 consecutive patients (187 women, 57 men; mean age, 68.2 years; standard deviation, 11.3 years; age range, 20-98 years) were included in this study. Most of the 244 patients presented with chronic low back pain, including only back pain (n = 88, 36.1%) and back pain with leg pain (n = 156, 63.9%).
The initial follow-up after an intra-articular facet joint steroid injection was conducted after 24.8 days on average (standard deviation, 19.3 days; range, 3-93 days). Responses according to the 5-point patient satisfaction scale are shown in Table 1. Improvement (including slightly improved, much improved, no pain) was seen in 208 patients (85.2%). Excellent improvement (including much improved, no pain) was seen in 168 patients (68.9%). In the postoperative patients, improvement (including slightly improved, much improved, no pain) was seen in 16 patients (80%). Excellent improvement (including much improved, no pain) was seen in 10 patients (50.0%). There was no statistically significant difference in the rate of pain improvement after an initial facet joint injection between the non-operative and postoperative groups (p = 0.51). In addition, the presence of leg pain was not statistically significant in the rate of pain improvement after an initial facet joint injection (p = 0.71).
Intra-articular facet joint steroid injections were repeated according to the patient's response and willingness. Most patients only underwent an initial intra-articular facet joint steroid injection (n = 189, 77.5%). Repeated intra-articular facet joint injections were given four times to 6 patients (2.5%), three times to 9 patients (3.7%), and twice to 40 patients (16.4%) until December 2007.
Among the 208 patients who showed improvement after a facet joint injection, 162 (77.9%, n = 162/208) showed symptom recurrence. The recurrence dates were grouped as shown in Table 2, which also includes those groups that experienced no recurrence and those with no improvement. The median symptom-free interval for cases that showed improvement after a facet joint injection was 69 days (95% confidence interval 52.2-85.8 days). Of the total 244 patients, 32% (n = 78) reported fewer than 60 days of pain relief, 53.3% (n = 130) reported more than 60 days pain relief, and 14.8% (n = 36) reported no pain relief. Overall, 30.3% reported a symptom-free interval of more than 6 months. In the postoperative patients, 50.0% (n = 10) reported fewer than 60 days of pain relief, and 30.0% (n = 6) reported more than 60 days of pain relief. Only 20.0% reported a symptom-free interval of more than 6 months.
The total numbers of intra-articular facet joint steroid injections per patient administered from 2007 to December 2009 are shown in Table 3. The mean total numbers of facet joint injections per patient was 2.1 in the 244 patients (standard deviation, 1.96; range, 1-15) during this period. Most patients underwent intra-articular facet join steroid injection fewer than five times (223/244, 91.4%) from 2007 to 2009.
In the 320 intra-articular facet joint steroid injections, all facet joint injections were successful under fluoroscopic guidance [partial success (n = 15, 4.7%) and complete success (n = 305, 95.3%)], according to the fluoroscopic arthrogram findings.
The presence of epidural leakage was found in fluoroscopic images of 74 (33.3%) of the 222 facet joint injection cases. The responses and the duration of symptom relief after facet joint injections according to the presence of epidural leakage are shown in Table 4. There were no significant differences in the response and duration of symptom relief after facet joint injections between two groups.
Our results showed that approximately 85% of patients had improvement after an initial intra-articular facet joint steroid injection; approximately 69% of the patients showed excellent improvement after the initial injection. On the other hand, approximately 78% of the patients showed symptom recurrence, with a median symptom-free interval of 69 days, while approximately 30% of the patients showed a symptom-free interval of more than 6 months. One third of the intra-articular facet joint injections showed epidural contrast leakage.
According to a study by Destouet et al. (17), among 54 patients who underwent intra-articular facet joint injection, 54% (n = 29/94) immediately responded to the injection, 20% (n = 11/54) had prolonged relief, and only 11% (n = 6/54) remained free of pain for 6-12 months. Carette et al. (24) suggested that about 22% (n = 11) of 49 patients showed sustained improvement for 6 months following one intra-articular fact joint injection. Gorbach et al. (19) reported that about 74% (n = 31) of 42 patients had an immediate positive response to the intra-articular facet joint injection, whereas only 34% (n = 14/42) of these patients exhibited a positive effect after 3 months. Our results were better than those reported previously: 85% of our patients reported an immediate response, approximately 53% had prolonged pain relief (> 2 months), and 30% remained free of pain for more than 6 months. These results could be due to variety of factors including patient selection bias, the precise injection technique under fluoroscopy, and checking the intra-articular location of the needle by arthrography.
Lynch and Taylor (25) suggested that the intra-articular facet joint injection is more effective than the pericapsular injection. Obtaining a better therapeutic effect for the intra-articular injection was theoretically thought to be important because the facet joint pain may be caused by synovitis inside the joint (252627). Based on the previous studies that demonstrated the involvement of inflammatory mediators in degenerative facet joints to explain facet joint pain, we suggest that intra-articular facet joint steroid injections may provide intermediate-term pain relief in those patients whose pain is accompanied by an active inflammatory process (17282930).
This study included the intra-articular facet joint injection of postoperative patients. The immediate effectiveness of this injection was similar to that of the intra-articular facet joint injection in the total patients. In addition, the symptom-free interval of the postoperative patients was similar to that of the total patients.
The success rate of the intra-articular facet joint steroid injection under fluoroscopic guidance was excellent (100%) in our study. These results demonstrate that we could easily approach the intra-articular space of the facet joint under fluoroscopy guidance.
According to our study, epidural leakage was found frequently during intra-articular facet joint steroid injection. To our knowledge, only two studies have previously reported the occurrence of epidural leakage during such an injection. Shih et al. (31) and Schulte et al. (16) reported an incidence of approximately 1% (n = 3/39 and n = 3/277) on the basis of clinical and radiological findings, respectively. However, our study suggested that the incidence of epidural leakage during intra-articular facet joint injection was approximately 33%. Epidural leakage of the injectate could result from the rupture of the facet joint capsule caused by the intra-articular facet joint injection. In addition, our study showed that there was no significant difference in the response and duration of symptom relief after facet joint injections according to the presence of epidural leakage.
This common epidural leakage in intra-articular facet joint injection means that intra-articular facet joint steroid injections could approach to the epidural space and have the effectiveness of an epidural injection. In cases of high-risk patients who have bleeding tendencies, severe spinal stenosis, or severe neural foraminal stenosis, the leakage of an intra-articular facet joint steroid injection under fluoroscopy might prove to be an easier and safer technique than a direct epidural steroid injection. The effectiveness of epidural leakage during an intra-articular facet joint injection could be expected to be similar to that of epidural injection. We could suggest that the epidural leakage of intra-articular facet joint injection is worthy of consideration, especially in high-risk patients who have bleeding tendencies. Also, we suggest that a triamcinolone acetonide suspension could be used for the intra-articular facet joint injection despite the epidural leakage of the intra-articular facet joint injection. Epidural leakage of the intra-articular facet joint injection could not cause direct epidural vessel penetration or vascular injury, which result in epidural hematoma or intra-arterial injection of steroid suspension. Therefore, epidural leakage of the intra-articular facet joint injection is unlikely to cause complications such as epidural hematoma or spinal cord ischemia. Fluoroscopy is then helpful simply for accessing the intra-articular facet joint space and confirming epidural leakage during the intra-articular facet joint injection.
Our study had the following limitations. Because we restrospectively analyzed the original medical records and fluoroscopic arthrograms, we could not form a control group receiving a placebo treatment or design a regular follow-up. However, the presence and extent of the placebo effect have to be taken into account when discussing the efficacy of facet joint injections (1932). In addition, we thought that the presence of the patients' symptoms was more important than regular follow-ups. Facet joint pain was diagnosed on the basis of clinical findings in our study because we assumed that intra-articular facet joint steroid injection has diagnostic and therapeutic implications. In addition, if we included only patients who were diagnosed by a diagnostic facet joint block, the study would have shown better results. Thirdly, we could not analyze the effectiveness of epidural leakage and investigate the conservative treatment of patients, such as with medication or physical therapy, because the characteristics of the patients who were included in this study were heterogeneous. We suggest that a control study is needed. In addition, the leakage to the back muscles could not be included in our assessment of the epidural leakage of the facet joint injection because we retrospectively reviewed the fluoroscopic images and the leakage to the back muscles is difficult to differentiate from the diagnostic contrast injection during the approach to the facet joints.
In conclusion, this study showed that fluoroscopy-guided intra-articular facet joint injection exhibits excellent immediate effectiveness and good prolonged pain relief (> 2 months) in patients with chronic low back pain; moreover, it is a reliable and easy technique for the management of low back pain. Epidural leakage during intra-articular facet joint injection was detected in approximately one-third of the cases.
Figures and Tables
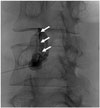 | Fig. 1A 35-year-old woman undergoing an intra-articular facet joint steroid injection at the left side L3/4 level. The fluoroscopic image shows an intra-articular sigmoid linear filling pattern (arrows). |
 | Fig. 2A 75-year-old man underwent intra-articular facet joint steroid injection from both sides at the L4/5 level.
A. A fluoroscopic image of the left side L4/5 facet joint injection shows the intra-articular arthrographic pattern.
B, C. On oblique (B) and anteroposterior (C) fluoroscopic images of the ipsilateral side L4/5 facet joint injection, there is contrast leakage into the epidural space (arrows).
|
Table 1
Response after an Initial Intra-Articular Facet Joint Steroid Injection

Table 2
Follow-Up and Recurrence after a Serial Intra-Articular Facet Joint Steroid Injection

Table 3
Total Numbers of Intra-Articular Facet Joint Steroid Injections Administered from 2007 to December 2009

| Total Number of Facet Joint Injection | Frequency (%) |
|---|---|
| 1 | 135 (55.3) |
| 2 | 50 (20.5) |
| 3 | 23 (9.4) |
| 4 | 15 (6.1) |
| 5 | 7 (2.9) |
| > 5 (6-15) | 14 (5.7) |
| Total | 244 (100) |
Table 4
Response and Recurrence According to the Presence of Epidural Leakage after Intra-Articular Facet Joint Steroid Injections

References
1. Boswell MV, Colson JD, Sehgal N, Dunbar EE, Epter R. A systematic review of therapeutic facet joint interventions in chronic spinal pain. Pain Physician. 2007; 10:229–253.
2. Sehgal N, Shah RV, McKenzie-Brown AM, Everett CR. Diagnostic utility of facet (zygapophysial) joint injections in chronic spinal pain: a systematic review of evidence. Pain Physician. 2005; 8:211–224.
3. Slipman CW, Bhat AL, Gilchrist RV, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003; 3:310–316.
4. Manchikanti L, Singh V, Pampati V, Damron KS, Barnhill RC, Beyer C, et al. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician. 2001; 4:308–316.
5. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976). 1994; 19:1132–1137.
6. Schwarzer AC, Wang SC, O'Driscoll D, Harrington T, Bogduk N, Laurent R. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine (Phila Pa 1976). 1995; 20:907–912.
7. Datta S, Lee M, Falco FJ, Bryce DA, Hayek SM. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician. 2009; 12:437–460.
8. Sehgal N, Dunbar EE, Shah RV, Colson J. Systematic review of diagnostic utility of facet (zygapophysial) joint injections in chronic spinal pain: an update. Pain Physician. 2007; 10:213–228.
9. Manchikanti L, Manchikanti KN, Manchukonda R, Cash KA, Damron KS, Pampati V, et al. Evaluation of lumbar facet joint nerve blocks in the management of chronic low back pain: preliminary report of a randomized, double-blind controlled trial: clinical trial NCT00355914. Pain Physician. 2007; 10:425–440.
10. Fuchs S, Erbe T, Fischer HL, Tibesku CO. Intraarticular hyaluronic acid versus glucocorticoid injections for nonradicular pain in the lumbar spine. J Vasc Interv Radiol. 2005; 16:1493–1498.
11. Murtagh FR. Computed tomography and fluoroscopy guided anesthesia and steroid injection in facet syndrome. Spine (Phila Pa 1976). 1988; 13:686–689.
12. Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine (Phila Pa 1976). 2007; 32:1754–1760.
13. Bogduk N. A narrative review of intra-articular corticosteroid injections for low back pain. Pain Med. 2005; 6:287–296.
14. Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006; 15:Suppl 2. S192–S300.
15. Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976). 2009; 34:1078–1093.
16. Schulte TL, Pietilä TA, Heidenreich J, Brock M, Stendel R. Injection therapy of lumbar facet syndrome: a prospective study. Acta Neurochir (Wien). 2006; 148:1165–1172. discussion 1172
17. Destouet JM, Gilula LA, Murphy WA, Monsees B. Lumbar facet joint injection: indication, technique, clinical correlation, and preliminary results. Radiology. 1982; 145:321–325.
18. Lau LS, Littlejohn GO, Miller MH. Clinical evaluation of intra-articular injections for lumbar facet joint pain. Med J Aust. 1985; 143:563–565.
19. Gorbach C, Schmid MR, Elfering A, Hodler J, Boos N. Therapeutic efficacy of facet joint blocks. AJR Am J Roentgenol. 2006; 186:1228–1233.
20. Revel ME, Listrat VM, Chevalier XJ, Dougados M, N'guyen MP, Vallee C, et al. Facet joint block for low back pain: identifying predictors of a good response. Arch Phys Med Rehabil. 1992; 73:824–828.
21. Peh W. Image-guided facet joint injection. Biomed Imaging Interv J. 2011; 7:e4.
22. Boswell MV, Shah RV, Everett CR, Sehgal N, McKenzie Brown AM, Abdi S, et al. Interventional techniques in the management of chronic spinal pain: evidence-based practice guidelines. Pain Physician. 2005; 8:1–47.
23. Lee JW, Shin HI, Park SY, Lee GY, Kang HS. Therapeutic trial of fluoroscopic interlaminar epidural steroid injection for axial low back pain: effectiveness and outcome predictors. AJNR Am J Neuroradiol. 2010; 31:1817–1823.
24. Carette S, Marcoux S, Truchon R, Grondin C, Gagnon J, Allard Y, et al. A controlled trial of corticosteroid injections into facet joints for chronic low back pain. N Engl J Med. 1991; 325:1002–1007.
25. Lynch MC, Taylor JF. Facet joint injection for low back pain. A clinical study. J Bone Joint Surg Br. 1986; 68:138–141.
26. Sinclair DC, Feindel WH, Weddell G, Falconer MA. The intervertebral ligaments as a source of segmental pain. J Bone Joint Surg Br. 1948; 30B:515–521.
27. Mooney V, Robertson J. The facet syndrome. Clin Orthop Relat Res. 1976; (115):149–156.
28. Cohen SP, Raja SN. Pathogenesis, diagnosis, and treatment of lumbar zygapophysial (facet) joint pain. Anesthesiology. 2007; 106:591–614.
29. Willburger RE, Wittenberg RH. Prostaglandin release from lumbar disc and facet joint tissue. Spine (Phila Pa 1976). 1994; 19:2068–2070.
30. Igarashi A, Kikuchi S, Konno S, Olmarker K. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine (Phila Pa 1976). 2004; 29:2091–2095.
31. Shih C, Lin GY, Yueh KC, Lin JJ. Lumbar zygapophyseal joint injections in patients with chronic lower back pain. J Chin Med Assoc. 2005; 68:59–64.
32. Hróbjartsson A. The uncontrollable placebo effect. Eur J Clin Pharmacol. 1996; 50:345–348.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download