Abstract
Coexistent breast malignancy arising in phyllodes tumor is extremely rare, and most of them are incidental reports after surgical excision. Coexistent malignancy in phyllodes tumor can vary from in-situ to invasive carcinoma. Lobular neoplasia is separated into atypical lobular hyperplasia and lobular carcinoma in situ (LCIS). LCIS is known to have a higher risk of developing invasive cancer. We reported imaging findings of multifocal LCIS arising in benign phyllodes tumor.
Phyllodes tumor is a rare type of breast tumor accounting for < 0.3-0.5% of breast tumors in female. Additionally, coexistent breast carcinoma can occur in about 1-2% of the phyllodes tumors. Phyllodes tumors that coexist with other neoplasms are classified as external to or within the phyllodes tumor (1). Most of the reported cases are external to the phyllodes tumor. The within the phyllodes tumor cases have been rarely reported; however, the imaging findings were reported in a few studies. To the best of our knowledge, there were no reports dealing with MRI findings including spectroscopy. Therefore, we reported a case of multi-focal lobular carcinoma in situ (LCIS) arising in benign phyllodes tumor with its multimodality imaging. Imaging and clinical findings of this case were reviewed retrospectively. The review was approved by the Institutional Review Board of our insttution. Informed consent was waived.
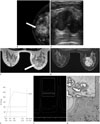
A 41-year-old woman was admitted for a palpable mass in the right breast that was first noted 3 days ago. She had no history of oral contraceptive/estrogen therapy, or family history of breast carcinoma. On physical examination, the mass was 4 cm, firm and movable involved the central location. Regardless of relatively large firm mass, there was no associated skin abnormality or axillary lymph node. Mammography demonstrated a 4.16 × 3.70 × 3.80 cm round mass with obscured margin and equal density at the 12 o'clock subareola of the right breast (Fig. 1A). We performed diagnostic breast ultrasonography, which revealed a 4.23 × 1.94 × 5.25 cm sized relatively oval mass with heterogeneous internal echo pattern (Fig. 1B). No significant vascularity was observed. She underwent breast biopsy for the mass that was diagnosed as benign phyllodes tumor.
Three weeks later, the patient underwent bilateral dynamic contrast-enhanced MRI to further evaluate the extent of the mass and the potential for combined malignancy. MRI revealed 4.9 × 3.6 × 3.7 cm, iso and high-signal intense oval mass on T2-weighted image and iso-intense on T1-weighted image, which showed heterogeneous enhancement with dark non-enhancing internal septations after contrast injection (Fig. 1C, D). The kinetics assessed by fully automated computer-aided detection-based analysis was initial fast (181%) followed by delayed washout (> 10%) (Fig. 1E). We could not exclude the possibility of the combined invasive malignancy since the mass had malignant kinetics.
MR spectroscopy (MRS) was performed on a Signa HD 1.5 T MR (GE Healthcare, Milwaukee, WI, USA) using the manufacturer's proton MRS acquisition software, breast spectroscopic examination. Before contrast injection, single-voxel, total choline containing compounds (tCho) spectrum was obtained within the mass to avoid contrast effect on the MRS (Fig. 1F). The presence of tCho in the mass was identified by a peak height at 3.2 ppm that was 2 fold higher than baseline noise. In addition, automatic choline quantification was 0.0161 mmol/L with the LCModel (LCModel Inc., Oakville, Canada).
The patient underwent conserving operation with sentinel lymph node biopsy. The pathology revealed multi-focal LCIS in a benign phyllodes tumor (Fig. 1G) and sentinel lymph nodes had no tumor.
Phyllodes tumor is a rare biphasic neoplasm composed of epithelial and stromal elements. It has characteristic leaf-like architecture, containing elongated cleft-like spaces and papillary projections of epithelial-lined stroma. In phyllodes tumor, malignant transformation usually occurs in the stromal component, therefore histologic features of stromal cellular atypia, mitotic activity, stromal overgrowth, and tumor margins are used for differentiating malignant from benign phyllodes tumor.
Almost all epithelial changes in phyllodes tumors are benign, although in situ and invasive carcinoma has rarely been reported (2345). To date, 35 phyllodes tumors with coexistent breast cancer have been reported in the literature. Of them, benign phyllodes were 19 cases, malignancies were 12, and the others were 3 borderline and 1 unknown case. Of benign phyllodes tumors, 11 in-situ (7 DCIS, 2 LCIS, 2 DCIS and LCIS) and 8 invasive coexistences (5 ductal, 2 lobular, 1 squamous cell carcinoma) were developed. Of malignant phyllodes tumors, 7 in-situ (6 DCIS, 1 LCIS) and 5 invasive coexistences (3 ductal, 2 squamous cell carcinomas) were developed. Of 3 borderline phyllodes tumors, DCIS was 2 cases and LCIS with tubular carcinoma was 1 case. One unknown phyllodes tumor had squamous cell carcinoma. These reported cases suggest that LCIS is less frequent than DCIS, invasive ductal carcinomas. Our case is interesting because the combined tumor was multi-focal LCIS (2345).
Etiologic relationship between phyllodes tumors and various carcinomas is still unclear; however, it occasionally occurs in the mammary gland near the phyllodes tumor. Some studies showed that it is induced by the stromal component of phyllodes tumor (46). Results from another study suggested that systemic growth factors and hormones are responsible for the carcinoma (6). In that case, carcinogenesis can show multicentricity and occur outside the phyllodes tumor, therefore secondary involvement of epithelium in phyllodes tumor is usual. Consequently, we considered our case as stromal change of phyllodes tumor because LCIS was within the phyllodes tumor.
Phyllodes tumors usually show well-defined margins with a round or lobulated shape and inner septated structure (7), which is consistent with our findings. LCIS is not only a risk factor but may also be a direct precursor to cancer. Thus, surgical excision should be conducted. Because of rarity of coexistent tumor within phyllodes tumor, MR features have been reported.
Our case showed initial rapid enhancement and increased total choline compound. Actually, LCIS is not an invasive breast cancer. Therefore, its malignant kinetics and increased total choline compound could be confusing to differentiate from malignancy. However, a few benign tumors can have malignant kinetics and benign tumor such as proliferative fibroadenoma or normal breast tissue of lactating woman may also show a positive tCho signal (89).
Recommended treatment of phyllodes tumor includes wide local resection with margins of at least 1-2 cm without axillary node dissection since lymph node metastasis is rare (4). Adjuvant therapy such as chemotherapy or radiotherapy can be additionally used, but remains to be defined (4). There is no standardized treatment for combined neoplasm of phyllodes tumor, due to the limited reports.
In conclusion, we reported a very unusual case of multi-focal LCIS arising in benign phyllodes tumor with its MR image findings.
Figures and Tables
Fig. 1
A 41-year-old woman with multi-focal lobular carcinoma in situ in a phyllodes tumor.
A. A craniocaudal mammogram shows a 4.16 × 3.70 × 3.80 cm sized, round mass with obscured margin and equal density in right subareolar breast at 12 o'clock (arrow).
B. Breast ultrasonography shows relatively oval mass with heterogeneous echogenicity.
C. T2-weighted axial MRI shows iso- and high-signal intense oval mass (arrow).
D. Post-contrast T1-weighted MRI shows heterogeneous enhancement with dark non-enhancing internal septations (arrowheads) in the mass.
E. Kinetics demonstrate initial fast (181%) followed by delayed washout (> 10%) with a fully automated computer-aided detection-based analysis.
F. MR spectroscopy of the mass shows the presence of total choline containing compounds spectrum with a peak height at 3.2 ppm that was 2 fold above the baseline noise.
G. The mass depicts 5 × 4-cm, benign phyllodes tumor with less than 3 mitotic figure/10 HPF and clear resection margins (H&E stain, × 40). Multi-focal lobular carcinoma in situ involves the stromal components of benign phyllodes tumor (inlet, H&E stain, × 200).
H&E = hematoxylin and eosin
References
1. Shirah GR, Lau SK, Jayaram L, Bouton ME, Patel PN, Komenaka IK. Invasive lobular carcinoma and lobular carcinoma in situ in a phyllodes tumor. Breast J. 2011; 17:307–309.
2. Padmanabhan V, Dahlstrom JE, Chong GC, Bennett G. Phyllodes tumor with lobular carcinoma in situ and liposarcomatous stroma. Pathology. 1997; 29:224–226.
3. Nio Y, Iguchi C, Tsuboi K, Maruyama R. Ductal carcinoma in situ arising within a benign phyllodes tumor: a case report with a review of the literature. Oncol Lett. 2011; 2:223–228.
4. Nomura M, Inoue Y, Fujita S, Sakao J, Hirota M, Souda S, et al. A case of noninvasive ductal carcinoma arising in malignant phyllodes tumor. Breast Cancer. 2006; 13:89–94.
5. Shin DJ, Kim DB, Roh JH, Kwak BS. Ductal carcinoma in situ arising in a benign phyllodes tumor: a case report. J Korean Soc Radiol. 2013; 68:423–426.
6. Deodhar KK, Baraniya JB, Naresh KN, Shinde SR, Chinoy RF. Cancerization of phyllodes tumour. Histopathology. 1997; 30:98–99.
7. Tan H, Zhang S, Liu H, Peng W, Li R, Gu Y, et al. Imaging findings in phyllodes tumors of the breast. Eur J Radiol. 2012; 81:e62–e69.
8. Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999; 211:101–110.
9. Tse GM, Yeung DK, King AD, Cheung HS, Yang WT. In vivo proton magnetic resonance spectroscopy of breast lesions: an update. Breast Cancer Res Treat. 2007; 104:249–255.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download