Abstract
Immunoglobulin G4 (IgG4)-related disease is a well-known disorder characterized by an inflammatory reaction with an increase in the number of IgG4-positive plasma cells associated with sclerosis. IgG4-related disease often affects the dura mater with a pattern of diffuse thickening when the central nervous system is involved. However, some nodular dural thickening requires discrimination from tumors because of obviously different treatment options. We report of a case of IgG4-related disease with tumefactive dural involvement.
Immunoglobulin G4 (IgG4)-related disease is a well-known clinicopathological entity characterized by inflammation that can occur in multiple organ systems with predominance in elderly men in their 50s or 60s (1). IgG4-related disease has characteristic histological features, including lymphoplasmacytic inflammation, fibrosis, obliterative phlebitis, and an increased number of IgG4-positive plasma cells (2). This disease was first described in patients with autoimmune pancreatitis who also had extra-pancreatic diseases, such as biliary lesions, sialadenitis, retroperitoneal fibrosis, enlarged celiac and hilar lymph nodes, chronic thyroiditis, and interstitial nephritis (3).
IgG4-related disease often affects the dura mater as hypertrophic pachymeningitis with a pattern of diffuse thickening when the central nervous system (CNS) is involved (245). Nodular pachymeningial thickening can occasionally present as a mass-like lesion that can be confused with a tumor. The differential diagnosis of dural lesions includes infections and neoplasms including meningioma, lymphoma, and metastasis. It is important to discriminate IgG4-related disease from tumors because the former responds well to steroid therapy (4).
Early studies evaluating autoimmune pancreatitis tended to consider that IgG4-positive cell counts of > 10 cells/high powered field (HPF) to > 30 cells/HPF as sufficient for the diagnosis. However, no criteria have been established for diagnosing extra-pancreatic disease. Some studies on organs, such as the lung, lymph nodes, and salivary glands, advocate using the ratio of IgG4/IgG-positive cells to establish diagnostic cutoff levels. Ratios of 30-50% have been applied for diagnosing cases of extra-pancreatic IgG4-related disease. Therefore, a pathological confirmation is essential and anti-IgG4 antibody immunostaining is useful for the diagnosis (2).
Here, we describe the radiological features of a case of IgG4-related disease with tumefactive dural involvement and provide a comprehensive literature review of IgG4-related disease involving the dura mater. This report was approved by our Institutional Review Board.
A 57-year-old previously healthy man presented with a first-onset generalized tonic-clonic seizure. The initial non-enhanced brain computed tomography (CT) scan showed a slightly hyperdense mass along anterior falx cerebri (Fig. 1A). Magnetic resonance imaging (MRI) revealed intermediate signal intensity on T1- and low signal intensity on T2-weighted images with extensive perilesional parenchymal edema (Fig. 1B, C). A post-contrast T1-weighted image showed diffuse thickening and enhancement of dura mater at the frontal interhemispheric fissure and parasagittal region with involvement of the superior sagittal sinus. The mass showed homogeneous enhancement with a jagged border, and adjacent brain parenchymal enhancement was also detected (Fig. 1D). No significant diffusion restriction was observed on a diffusion-weighted image.
With suspicion of a meningioma, the patient underwent a frontal craniotomy, and the mass lesion was removed. Histologic examination revealed a mass with diffuse lymphoplasmacytic infiltration, severe fibrosis, and phlebitis. An immunohistochemical study showed up to 26 IgG4-positive cells per HPF, supporting the diagnosis of IgG4-related disease (Fig. 2). Serum IgG4 level was not available.
The patient was medicated with corticosteroid (250 mg/day methylprednisolone) for the next 6 days after surgery and was discharged on day 10 after surgery without any neurologic deficit. The last postoperative follow-up MRI on week 6 after discharge revealed significant improvement in the dural lesions.
Tumefactive dural involvement of IgG4-related disease was detected in this case that could be treated by surgical resection and corticosteroid administration. Intracranial involvement of IgG4-related disease is a relatively rare condition and the tumefactive presentation is even rarer. However, it is important to recognize IgG4-related conditions because they are medically treatable and respond well to corticosteroid therapy.
IgG4-related pachymeningitis was not previously a separate disease entity from idiopathic hypertrophic pachymeningitis. Linear thickening of the falx and tentorium is the most common finding on an imaging study of idiopathic hypertrophic cranial pachymeningitis, and the next most common finding is focal nodular thickening that simulates a dural mass (6). An increasing number of reports suggest that idiopathic hypertrophic pachymeningitis may be part of a disease spectrum, although not all such cases can be categorized as IgG4-related sclerosing disease (7). One report reviewed all pathology specimens diagnosed as noninfectious hypertrophic pachymeningitis over 25 years at their institution and IgG4-related disease represented 29% of all cases (4). Therefore, dural involvement of IgG4-related disease is a relatively rare condition (5) and tumefactive dural involvement is even rarer, as shown in our literature review in the following paragraphs.
We searched the PubMed database using the keywords: "IgG4-related disease & pachymeningitis" and "IgG4-related disease & dura mater" and found clinical and radiological information on 43 cases from 27 reports (58). The patients consisted of 27 males (63%) and 16 females (37%) with a mean age of 53.2 ± 11.6 years (range, 32-82 years). The cases of IgG4-related disease most commonly showed more than two sites of dural involvement (18 cases, 41.8%), including the convexity, posterior fossa, skull base, tentorium, or falx. Single lesions were more common at the convexity (13 cases, 30.2%) or the skull base (7 cases, 16.3%) (Table 1). MRI revealed relatively low signal intensity on T2-weighted images, representing fibrosis and intermediate signal intensity on T1-weighted image. Post-contrast T1-weighted images showed well-enhanced lesions with diverse features, including diffuse even dural thickening (17 cases, 39.5%) or diffuse dural thickening with nodularity (13 cases, 30.2%) (Table 2). Co-involvement of brain parenchyma was reported in 9 cases (20.9%) and co-involvement of leptomeninges was identified in 3 cases (6.9%).
In our case, the lesion was located in the dura mater encompassing the anterior falx and parasagittal convexity. A post-contrast T1-weighted image showed diffuse dural thickening with extreme nodularity that may resemble a tumor. The adjacent superior sagittal sinus and brain parenchyma also showed enhancement and extensive perilesional parenchymal edema disproportionate to its size on a T2-weighted image. The likelihood of IgG4-related disease rather than a meningioma is suggested by relatively more prominent and thicker hypertrophy of the dura mater than the usual dural tail sign associated with meningioma. This finding can be helpful to differentiate IgG4-related disease with tumefactive dural involvement from a meningioma.
The differential diagnosis should include conditions, such as lymphoma, metastasis, and infectious meningitis. We considered the possibility of dural lymphoma presenting as a dural mass. Although the mass was hyperdense on CT, reflecting a possible highly cellular nature of the lesion, it did not show any diffusion restrictions, so we excluded primary dural lymphoma.
A dural metastasis occurs occasionally and the most frequently reported primary neoplasms are in the prostate, kidney, and breasts. The macroscopic appearance of a dural metastasis is often indistinguishable from a meningioma. Therefore, a metastasis should be considered until proven otherwise in a patient with a dural mass and known systemic cancer (9). However, considering that our patient had no history of malignancy and showed no systemic symptoms associated with malignancy, we ruled out a dural metastasis.
Granulomatous diseases, such as fungal diseases, may produce dural masses and pachymeningeal enhancement. However, these granulomatous processes typically affect the basilar meninges, rather than the convexities of the cerebral hemispheres. The patient's clinical presentation provides clues for the differential diagnosis when fever or other signs of infection exist. A lumbar puncture may reveal pleocytosis, and cerebrospinal fluid cultures may demonstrate an organism (10). Our patient was immunocompetent and did not show any signs of infection or laboratory findings supporting a fungal infection diagnosis.
In conclusion, IgG4-related disease can demonstrate a dural-based mass in cases of CNS involvement. IgG4-related disease should be kept in the differential diagnosis because it has obviously different treatment options when a dural-based mass accompanies excessive hypertrophy of adjacent dura mater.
Figures and Tables
Fig. 1
Computed tomography (CT) and magnetic resonance imaging of immunoglobulin G4-related disease with tumefactive dural involvement in a 57-year-old man. Initial non-enhanced CT (A) demonstrates a slightly hyperdense mass (black arrow) along the anterior falx. The mass (black arrow) shows intermediate signal intensity on T1- (B) and low signal intensity on T2-weighted images (C) with extensive perilesional parenchymal edema. A post-contrast T1-weighted image (D) shows, diffuse thickening and enhancement of dura mater (white arrow) at the anterior falx cerebri and parasagittal region. The mass shows homogeneous enhancement with a jagged border and co-involvement of adjacent brain parenchyma (white arrowhead).
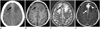
Fig. 2
Immunoglobulin G4 (IgG4) immunohistochemical staining (× 400) shows infiltration of abundant IgG4-positive plasma cells (white arrows).
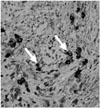
Table 1

| Involvement | Location | Patient Number (%) |
|---|---|---|
| Single location | Convexity | 13 (30.2) |
| Skull base | 7 (16.3) | |
| Posterior fossa | 4 (9.3) | |
| Tentorium | 1 (2.3) | |
| Two or more locations* | 18 (41.8) | |
| Total | 43 (100) |
Table 2
Radiologic Features in Immunoglobulin G4-Related Disease Involving Dura Mater on Postcontrast T1-Weighted Image in Literature Review (58)

References
1. Moss HE, Mejico LJ, de la Roza G, Coyne TM, Galetta SL, Liu GT. IgG4-related inflammatory pseudotumor of the central nervous system responsive to mycophenolate mofetil. J Neurol Sci. 2012; 318:31–35.
2. Lindstrom KM, Cousar JB, Lopes MB. IgG4-related meningeal disease: clinico-pathological features and proposal for diagnostic criteria. Acta Neuropathol. 2010; 120:765–776.
3. Lee LK, Sahani DV. Autoimmune pancreatitis in the context of IgG4-related disease: review of imaging findings. World J Gastroenterol. 2014; 20:15177–15189.
4. Wallace ZS, Carruthers MN, Khosroshahi A, Carruthers R, Shinagare S, Stemmer-Rachamimov A, et al. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore). 2013; 92:206–216.
5. Takeuchi S, Osada H, Seno S, Nawashiro H. IgG4-Related Intracranial Hypertrophic Pachymeningitis: a case report and review of the literature. J Korean Neurosurg Soc. 2014; 55:300–302.
6. Lee YC, Chueng YC, Hsu SW, Lui CC. Idiopathic hypertrophic cranial pachymeningitis: case report with 7 years of imaging follow-up. AJNR Am J Neuroradiol. 2003; 24:119–123.
7. D'Andrea G, Trillò G, Celli P, Roperto R, Crispo F, Ferrante L. Idiopathic intracranial hypertrophic pachymeningitis: two case reports and review of the literature. Neurosurg Rev. 2004; 27:199–204.
8. Lu LX, Della-Torre E, Stone JH, Clark SW. IgG4-related hypertrophic pachymeningitis: clinical features, diagnostic criteria, and treatment. JAMA Neurol. 2014; 71:785–793.
9. Hamid HA, Gee KY, Muhammad R, Abd Rahman ZA, Das S. Dural metastasis mimicking meningioma: an interesting case. Acta Medica (Hradec Kralove). 2009; 52:19–22.
10. Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007; 27:525–551.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download