INTRODUCTION
Inflammatory pseudotumor was first detected in the lung and described by Brunn in 1939. It was so named by Umiker and Iverson in 1954 because of its tendency to mimic a malignant process, clinically and radiologically (1). Inflammatory pseudotumor commonly involves the lung and the orbit, but it can occur in nearly every site in the body (2). Inflammatory pseudotumor has been reported in various sites in the abdomen, including the liver. Abdominal inflammatory pseudotumor should be included in the differential diagnosis of any soft-tissue mass within the abdomen and viscera (1). There are va-rious causes of inflammatory pseudotumor including secondary infection (2).
Perihepatitis is defined as inflammation of the peritoneal capsule of the liver and is classically described as being associated with pelvic inflammatory disease (PID) (the so-called Fitz-Hugh-Curtis syndrome). Fitz-Hugh-Curtis syndrome is thought to result from the peritoneal spread of infection from the pelvic cavity (3).
We report a case of inflammatory pseudotumor of the liver and omentum caused by PID, which shows the intraperitoneal spreading mechanism in Fitz-Hugh-Curtis syndrome.
CASE REPORT
A 47-year-old woman was admitted to our hospital for right sided abdominal pain. Physical examination showed a tender abdomen and slight rigidity in the right upper and lower quadrants. Body temperature was measured as 38.5℃.
Laboratory data of liver function parameters, such as aspartate aminotransferase (12 IU/L, normal range: 12-35 IU/L), alanine aminotransferase (12 IU/L, normal range: 7-35 IU/L), total bilirubin (0.15 mg/dL, normal range: 0.1-1.2 mg/dL) were within the normal range. Erythrocyte sedimentation rate (37 mm/hr, normal range: 0-20 mm/hr) and C-reactive protein (CRP, 8.57 mg/dL, normal range: 0.0-0.5 mg/dL) were elevated with leukocytosis [white blood cell (WBC) count 14220/mm3, normal range: 4400-11000/mm3].
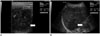
On abdominal ultrasound image, a 4 cm sized hypoechoic mass was detected in the right paracolic gutter (Fig. 1A). Also, a heterogenous hypoechoic lesion in liver segment #6 was observed (Fig. 1B). Bilateral tubo-ovarian abscesses were detected, with the left side being more severely affected.
In serial abdominal computed tomography scan (multidetector row CT, SOMATOM Sensation 64, Siemens Healthcare, Forchheim, Germany), bilateral tubo-ovarian abscesses with PID (Fig. 2A), a well-enhanced mass in the right paracolic gutter (Fig. 2B), and a heterogenously enhanced lesion in liver segment #6 with capsular retraction and enhancement were noted (Fig. 2C) [CT parameters: 120 kVp, 160 mAs, collimation 64 × 0.6 mm (with z-flying focal spot), and slice thickness 5 mm, Protocol: The images were taken in precontrast, arterial, portal, and delay-ed phases. Precontrast phase image was taken in the initial scan. Then, 1.5 mL per kg of contrast media was administered via intravenous bolus injection. Through bolus tracking, arterial phase image was taken when the Hounsfield unit (HU) value in the abdominal aorta exceeded 100 HU. Portal phase image was taken 50 seconds after bolus tracking, and the delayed phase image was taken 160 seconds after bolus tracking. The scan time for each phase was 6 seconds].
In positron emission tomography CT scan, intensely increased fluorodeoxyglucose uptake was noted in bilateral tubo-ovarian abscesses [maximum standardized uptake value (SUVmax): 8.16], right omental mass (SUVmax: 10.15) (Fig. 3), and mass-like lesion in liver segment #6 (SUVmax: 9.23).
We performed percutaneous ultrasound-guided biopsy for the mass in liver segment #6 (Fig. 4A) and the right omental mass (Fig. 4B). The images showed infiltration of lymphoplasma cells and some lymphocytes without cytologic atypia. The masses were pathologically confirmed to be inflammatory pseudotumors.
The patient underwent a two-week course of intravenous antibiotic treatment. The right sided abdominal pain and tenderness subsided, and the laboratory data also improved (WBC count 9700/mm3, CRP 0.26 mg/dL). The patient was discharged and she was lost to follow-up.
DISCUSSION
Inflammatory pseudotumors have been described under various names due to the variable sites of involvement; plasma cell granuloma (heart and lung), inflammatory myofibroblastic tumor (lung), inflammatory myofibrohistiocytic proliferation, histiocytoma, xanthoma, fibroxanthoma, fibrous xanthoma, xanthogranuloma, xanthomatous pseudotumor, plasma cell-histio-cytoma complex (lung), plasmacytoma, solitary mast cell gr-anulomas, and inflammatory fibrosarcoma (urinary bladder) (12).
Inflammatory pseudotumor may also occur in the abdominal cavity, which is an important mimicker of intraperitoneal tumor. Differentiation from malignant tumors can often be challenging because the radiologic findings of inflammatory pseudotumors are rather nonspecific. Abdominal inflammatory pseudotumor should be considered in the differential diagnosis of any soft-tissue mass within the abdomen and viscera. Percutaneous biopsy is the most reliable method for differential diagnosis, and it helps us to avoid unnecessary exploratory laparotomy or a hepatectomy in case of an uncertain diagnosis (12).
The causes of inflammatory pseudotumor are not known. Some authors claim, on the basis of multiple evidence, that it is a low-grade fibrosarcoma with inflammatory cells. The propensity of inflammatory pseudotumors to be locally aggressive, to frequently be multifocal, and to progress occasionally to a true malignant tumor supports this idea. In some cases, inflammatory pseudotumor is thought to result from inflammation following minor trauma or surgery, or to be associated with other malignancy (13).
There appears to be a subset of inflammatory pseudotumors that occur secondary to infection (1). Organisms found in association with inflammatory pseudotumor include mycobacteria associated with spindle cell tumor; Epstein-Barr virus found in splenic and nodal pseudotumors; actinomycetes and nocardiae found in hepatic and pulmonary pseudotumors, respectively; and mycoplasma in pulmonary pseudotumors (14). There have been case reports of inflammatory pseudotumor associated with infections caused by other organisms, including Mycobacterium avium-intracellulare complex, Corynebacterium equi, Escherichia coli, Klebsiella, Bacillus sphaericus, Pseudomonas, Helicobacter pylori, and Coxiella burnetti (13456).
PID refers to infection and resultant inflammation of the upper female genital tract, including the endometrium, fallopian tubes, and ovaries. PID is the result of ascending infection from the vagina and cervix; the most common organisms are Neisseria gonorrhoeae and Chlamydia trachomatis. Polymicrobial infection can occur in 30-40% of cases; tuberculosis and actinomycosis occur much less frequently (7).
In advanced PID patients, tubo-ovarian abscess and perihepatitis can develop. Perihepatitis is classically described as being associated with PID, the so-called Fitz-Hugh-Curtis syndrome (8). In Fitz-Hugh-Curtis syndrome, bacteria spread by means of direct extension along the right paracolic gutter or through the lymphatic system, causing inflammation of the right upper qu-adrant peritoneal surfaces and the right lobe of the liver (789).
Inflammation of the liver capsule is not visible on ultrasound (7). In dynamic CT, Fitz-Hugh-Curtis syndrome has been reported to manifest as intense enhancement along the anterior surface of the liver (79). The capsular enhancement seen in early-phase images may reflect increased blood flow in the inflam-ed hepatic capsule. On enhanced MR, dynamic postcontrast images show subcapsular and periportal areas of hypervascularity in the arterial phase. These areas are isointense relative to the rest of the hepatic parenchyma with delayed postcontrast se-quences (7).
In this case report, the patient complained of lower and right sided abdominal pain with fever along with laboratory findings that suggested an infection. CT scan of the patient after admission confirmed the diagnosis of PID. The patient was referred to the gynecologist and colposcopy showed prominent cervical motion tenderness. CT scan also showed multiple mass-like lesions in the right omentum and liver segment #6. These lesions were pathologically confirmed as inflammatory pseudotumors.
As already mentioned, the causes of inflammatory pseudotumor are not fully known, and they are rather vague and diverse. An infection with subsequent inflammation can be one of the causes. In this case, PID was confirmed through symptoms, signs, laboratory findings, and radiologic findings, although the pathogen responsible for PID was not clearly identified (10). An inflammatory pseudotumor was found in the right omentum and liver segment #6, which corresponds to the intraperitoneal spreading pathway involved in Fitz-Hugh-Curtis syndrome.
Tubo-ovarian abscess in the left adnexa appears to be a cystic lesion, whereas the lesions in the right omentum and the liver appear to be of solid nature; therefore, it is debatable as to whether these lesions have a common origin. But it is known that inflammatory lesions are observed in various forms during the course of their progression. Adnexal lesions, especially, are tubular st-ructures which can appear as cystic lesions (like tubo-ovarian abscess) (7). In addition, the patient had no previous trauma or operation history, comorbid malignancy, or infection other than PID. Therefore, this case serves as evidence supporting the known intraperitoneal spreading mechanism in Fitz-Hugh-Curtis syndrome, through a series of consequences such as PID and inflammatory pseudotumor.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download