Abstract
Purpose
To analyze ultrasonographic (US) features of metastatic lymph nodes (LNs) in papillary thyroid microcarcinomas (PTMC) and in papillary thyroid macrocarcinomas.
Materials and Methods
The study reviewed US findings of 273 patients with pathologically confirmed papillary thyroid carcinoma (PTC) and metastatic LNs based on the US examination. Patients were divided into two groups: PTMC and papillary thyroid macrocarcinomas.
Results
The 273 patients with PTC included 87 with PTMC and 186 with papillary thyroid macrocarcinoma. No significant difference of US features in patients with lateral neck node metastasis was found between PTMC (n = 96) and macrocarcinoma (n = 29). In central neck node metastasis, round shape was the most frequent findings in both groups (p < 0.001).
A papillary thyroid microcarcinoma (PTMC) is defined by the World Health Organization as a carcinoma measured at 1.0 cm or less in its greatest dimension (1). There is an increase in the proportion of PTMC identified among all differentiated thyroid carcinomas mainly due to the improvement and increased in use of ultrasound (US) examination, fine-needle aspiration biopsy, and other diagnostic procedures (2). It is estimated that PTMC accounts for up to 30% of all papillary thyroid cancers, although marked geographic differences in incidence rates have been noted (3). In South Korea, PTMC was reported in up to 50.2% of thyroid carcinomas (4). Currently, there is controversy on the extent of surgery necessary for PTMC. In published clinical guidelines of organizations including the American Thyroid Association, the National Comprehensive Cancer Network, and the British Thyroid Association, the general recommendation in cases of lymphadenopathy is to perform lymph node dissection of the affected compartments. In patients with biopsy-proven metastatic lateral cervical lymphadenopathy, therapeutic lateral neck compartmental lymph node dissection was recommended with total thyroidectomy (TT) to provide clearance of disease (5, 6, 7, 8). Patients with preoperatively detected lateral neck node metastasis are more likely to develop recurrence in the lymph nodes (9). Thus, preoperative US examination is important for evaluating lateral metastatic lymph nodes to determine the extent of operation in potentially aggressive PTMC.
Previous studies have reported the usefulness of preoperative US in detecting the presence of clinically apparent cervical lymph node metastasis (9, 10, 11). Ito et al. (9) reported that preoperative US could detect 39% of metastases among patients with pathologically confirmed lateral lymph node metastasis. However, no report has described whether there are any characteristic or dominant imaging features of metastatic lymph nodes in PTMC in comparison with those of papillary thyroid macrocarcinomas. Therefore, this study retrospectively reviewed the sonographic features of metastatic lymph nodes in PTMC compared to papillary thyroid macrocarcinomas and determined whether there were any dominant findings of lateral metastatic lymph nodes in PTMC. Additionally, this study investigated the sonographic features of metastatic lymph nodes limited to the central neck in PTMC and papillary thyroid macrocarcinoma.
From January 2009 through May 2012, 676 patients with pathologically confirmed papillary thyroid carcinoma (PTC) were identified by the Institutional Review Board. None of these patients had undergone previous operation on the head or neck. All patients underwent US before surgery.
Thyroid US was performed using a 5- to 12-MHz linear transducer (iU22; Philips Healthcare, Bothell, WA, USA) by one radiologist with ten years of experience. When US examination was performed for detecting metastatic lymph nodes, the radiologist was unaware of cytologic confirmation of malignancy in the thyroid masses. The US examinations included both thyroid lobes and all neck levels (I-VI).
Patients were placed into two groups depending on the size of the tumor. Patients placed in the PTMC group (n = 306) had tumors with a maximum diameter of 10 mm, and those placed in the papillary thyroid macrocarcinoma group (n = 370) had tumors greater than a minimum diameter of 1 cm. A total of 87 patients from the PTMC group and 186 patients from the papillary thyroid macrocarcinoma group were selected because they were suspected of metastatic lymph nodes based on US examination.
The US features of metastatic lymph nodes of the total 273 patients were reviewed by two radiologists. The following US criteria were used to define lymph node metastasis: round shape (minor axis greater than 50% of major axis), loss of fatty hilum, focal or diffuse hyperechogenicity, cystic change, and microcalcification (12, 13, 14). Initially, we compared US features of metastatic lymph nodes in the lateral neck of patients with PTMC and those of patients with papillary thyroid macrocarcinoma. Subsequently, the US features of metastatic lymph nodes in the central neck were reviewed.
Based on findings of US examinations, patients were classified into two groups: the first group had only central neck node (level VI) metastasis; the second group had metastases extended to the lateral neck node (levels II-V). The definition of cervical compartment used in this study was based on the rules outlined by the Head and Neck Service of the Memorial Sloan-Kettering Cancer Center (15, 16).
Of the 186 patients with papillary thyroid macrocarcinoma, 90 were suspected of only central neck node metastasis, whereas 96 were suspected of central and lateral neck node metastasis. Of the 87 patients with PTMC, 58 were suspected of only central neck node metastasis, while 29 were suspected of central and lateral neck node metastasis.
Lateral neck node metastasis was confirmed by Nodal fine-needle aspiration biopsy (FNAB) or Nodal FNAB needle-wash thyroglobulin (Tg). Nodal FNAB needle-wash Tg measurements could complement cytology in thyroid cancer because it has been reported that these measurements could be used as diagnostic substitute (17, 18). All thyroid tumors and lymph node metastases were confirmed as PTC by pathologic examination. In addition, lymph node metastases were identified by levels. Radiologic findings of suspected lymph node metastasis were correlated with the pathologic evaluation findings.
Patient flow diagram is shown in Fig. 1. All patients underwent routinely TT with central compartment neck dissection. Modified radical neck dissection (MRND) of the affected side was performed for patients diagnosed with extended lateral neck node metastasis (PTMC, n = 29; papillary thyroid macrocarcinoma, n = 96).
Statistical analysis was performed using the chi-square test and a Fisher's exact test. Statistical calculations were performed using the statistical package SPSS for Windows, released in 20.0.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was considered when p value was less than 0.05.
Of the 273 patients suspected of metastatic lymph nodes based on the US examination, 231 (84.6%) were women, 42 (15.4%) were men. The mean age was 45 years (range, 19-81 years).
The US features of metastatic lymph nodes in PTMC and papillary thyroid macrocarcinoma are summarized in Table 1. The following diagnostic results were found in the papillary thyroid macrocarcinoma group: round shape (central vs. lateral neck node metastasis: n = 86, 95.6% vs. n = 70, 72.9%), loss of hilum (n = 29, 32.2% vs. n = 84, 87.5%), hyperechogenicity (n = 14, 15.6% vs. n = 87, 90.6%), cystic change (n = 12, 13.3% vs. n = 32, 33.3%), and calcification (n = 2, 2.2% vs. n = 20, 20.8%). For the PTMC group, the following results were obtained: round shape (central vs. lateral neck node metastasis: n = 54, 93.1% vs. n = 19, 65.5%), loss of hilum (n = 12, 20.7% vs. n = 26, 89.7%), hyperechogenicity (n = 8, 13.8% vs. n = 27, 93.1%), cystic change (n = 4, 6.9% vs. n = 9, 31%), and calcification (n = 1, 1.7% vs. n = 7, 24.1%) (Figs. 2, 3, 4, 5).
Of the 125 patients suspected of lateral neck node metastasis, no significant difference of US features was found between the microcarcinoma (n = 96) and macrocarcinoma (n = 29) groups as shown in Figs. 4 and 5. The following analysis was made based on image findings: round shape (microcarcinoma vs. macrocarcinoma: n = 19, 65.5% vs. n = 70, 72.9%, p = 0.221), loss of hilum (n = 26, 89.7% vs. n = 84, 87.5%, p = 0.556), hyperechogenicity (n = 27, 93.1% vs. n = 87, 90.6%, p = 0.555), cystic change (n = 9, 31.0% vs. n = 32, 33.3%, p = 0.533), calcification (n = 7, 24.1% vs. n = 20, 20.8%, p = 0.435). The frequency of lateral neck node metastasis in papillary thyroid macrocarcinoma (52%) was significantly (p = 0.020) higher than that in PTMC (33%) (Table 2). There was significant difference of frequency in PTMC between central neck node (n = 58) and lateral neck node (n = 29) metastases, with p < 0.001 for loss of hilum, hyperechogenicity, cystic change, and calcification. Similar results were obtained for the papillary thyroid macrocarcinoma group with p < 0.001 for loss of hilum, hyperechogenicity, cystic change, and calcification. The round shape was the more frequent finding than other four variables in central neck node metastasis in both papillary thyroid macrocarcinoma group and PTMC group, with p < 0.001 for loss of hilum, hyperechogenicity, cystic change, and calcification (Table 3).
Currently research has indicated that thyroid cancer is the most common endocrine malignancy. PTC is the most frequent histological subtype, accounting for 80% of all cases. Increasing global interest in this disease partially stems from the increasing number of its diagnoses. Several authors have demonstrated that the incidence of PTC has almost doubled over the last three decades, mainly due to the higher incidence of PTMC (19, 20). This increase is attributable to the widespread use of cervical ultrasound and ultrasound-guided fine-needle aspiration biopsies of thyroid nodules as well as more accurate histopathological search for small PTMC (19, 21).
In spite of the overall excellent prognosis for patients with PTMC, there is a 1.0% disease-related mortality rate, 5.0% lymph node recurrence rate, and 2.5% distant metastasis rate associated with PTMC. In addition, PTMC has been highly associated with lymph node metastasis at the time of diagnosis, with incidence rate up to 26.3% (22). The presence of lymph node metastasis is an important prognostic factor that indicates the potential for an increasing rate of distant metastasis and the risk of cervical lymph node recurrence (3). Many factors such as tumor mutifocality, bilaterality, capsule invasion, and size (> 5 mm) have been proposed as risk factors of cervical lymph node metastasis in PTMC. These factors are the same for individuals with papillary thyroid macrocarcinoma (22, 23, 24). Some studies have demonstrated that distinguishing PTMC from conventional PTC based on size alone may be erroneous because the recurrence rates for PTMC and conventional PTC are not significantly different. In fact, both tumors behave similarly (25). Some authors have demonstrated that the ipsilateral lateral compartment was involved almost as often as the central compartment (24, 26, 27, 28). However, currently no report has compared any characteristic or dominant imaging features of metastatic lymph nodes in PTMC with papillary thyroid macrocarcinomas. The results from this study indicated that there were no significant difference in US features of metastatic lymph nodes between PTMC and papillary thyroid macrocarcinomas. Not only relatively high incidence of lymph node metastasis, but also all features of metastatic lymph nodes were well presented in PTMC examination. Treatment of patients with PTMC has been suggested to be similar to treatment of patients with conventional PTC (3, 29). Given the relatively low sensitivity of US in evaluating potential metastatic lymph nodes in the central neck, it is reasonable to consider a prophylactic central neck dissection with a total thyroidectomy or lobectomy, even when clear US evidence for local metastatic disease is absent. It is reasonable to consider a therapeutic central neck dissection based on intraoperative findings of positive nodal metastases in this region. Cervical nodal metastases are quite common in PTC. Initial nodal metastasis in PTC usually occurs in the paratracheal and pretracheal nodes in level VI of the central compartment of the ipsilateral neck (26). Preoperative B-Raf proto-oncogene analysis by FNAB based on US may assist the prediction of occult central neck lymph node metastasis in patients with PTC. Previous studies have used indications of clinically node-negative neck and tumor size > 5 mm as predictive factors for subclinical central lymph node metastasis in PTMC (30, 31). Many trials have used it to predict and detect central metastasis. However, the characteristic US features of central metastatic lymph nodes have not yet been demonstrated. Therefore, the US features of metastatic central neck lymph nodes were reviewed in this study to determine if there were any dominant findings that could be used clinically for central neck lymph node examinations. Our results indicated that round shape was the most frequent finding in central neck node metastasis of both PTMC and papillary thyroid macrocarcinomas. This might be due to the relatively low sensitivity of US in evaluating potential metastatic lymph nodes in the central neck. A considerably high percentage of small lesions might have led to the difficulty in identifying inner structures and any changes of lesions. Our findings also indicated that the possibility of metastasis might be suspected when the central lymph node exhibited only round shape.
This study had several limitations. First, only patients suspected of lateral neck node metastasis based on US and those who had undergone surgery with pathologically confirmed metastasis were included, which might have selection bias. However, based on research findings, MRND is not necessary nor recommended for patients without definite lateral neck node metastasis detected on preoperative US. This was because lymph node recurrence-free survival rates did not differ between patients who underwent MRND and patients who did not undergo MRND if there was no preoperative detection of lateral neck node metastasis. In addition, research has shown that, in more than 60% of patients, latent lymph node metastasis only occasionally becomes clinically apparent that requires treatment (9). Finally, this study analyzed only central neck nodes detected with US, which may cause selection bias. To overcome these limitations, further studies are needed to assess the findings using large study groups.
In conclusion, there was no significant difference in US findings of metastatic LNs between the PTMC and the papillary thyroid macrocarcinoma groups, although the frequency of lateral neck node metastasis in the macrocarcinoma group was higher than in the PTMC group. Therefore, it is recommended that careful evaluation of metastatic lymph nodes should be conducted, even if only microcarcinoma is present in the thyroid gland.
Figures and Tables
Fig. 1
Patient flow diagram. Flowchart shows the study population and included patients. Of 676 patients with pathologically confirmed papillary thyroid carcinoma, 186 patients from the papillary thyroid macrocarcinoma group and 87 patients from the PTMC group were selected because they were suspected of having metastatic lymph nodes based on the US examination.
Note.-LN = lymph node, PTMC = papillary thyroid microcarcinoma, US = ultrasonographic

Fig. 2
Central neck node metastasis in 56-year-old man.
A. Ultrasonographic image shows ill-defined hypoechoic papillary thyroid microcarcinoma with calcifications in the right thyroid gland.
B. An oval shaped lymph node at left level VI shows focal hyperechogenicity (arrow) with loss of hilum in the left central neck on ultrasonography.
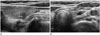
Fig. 3
Central neck node metastasis in 35-year-old woman.
A. Ultrasonographic image shows a lobulated margin, marked hypoechoic papillary thyroid macrocarcinoma with extracapsular extension in the right thyroid gland.
B. A round shaped lymph node (arrow) shows internal cystic change in the left central neck on ultrasonography.
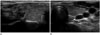
Fig. 4
Lateral neck node metastasis in 56-year-old man.
A. Ultrasonographic image shows a spiculated margin, marked hypoechoic papillary thyroid microcarcinoma in the right thyroid gland.
B. An oval shaped lymph node (arrow) shows loss of hilum, hyperechogenicity, and microcalcification in the left lateral neck on ultrasonography.
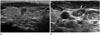
Fig. 5
Lateral neck node metastasis in 61-year-old woman.
A. Ultrasonographic image shows a spiculated margin, marked hypoechoic papillary thyroid macrocarcinoma with extracapsular extension in the right thyroid gland.
B, C. The right level III (B) and level IV (C) metastatic lymph nodes show microcalcification (white arrow), loss of hilum, and hyperechogenicity (black arrow) on ultrasonography.

Table 1
US Features of Metastatic LNs of 273 Patients

Table 2
US Features of Lateral Metastatic Lymph Nodes in Papillary Thyroid Macrocarcinoma and PTMC

Table 3
US Features of Central Metastatic Lymph Nodes in Papillary Thyroid Macrocarcinoma and PTMC

References
1. Hedinger CE, Williams ED, Sobin LH. Histologic typing of thyroid tumours. 2nd ed. Berlin: Springer-Verlag Berlin Heidelberg;1988. p. 9–10.
2. Baudin E, Travagli JP, Ropers J, Mancusi F, Bruno-Bossio G, Caillou B, et al. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998; 83:553–559.
3. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003; 98:31–40.
4. Lee J, Rhee Y, Lee S, Ahn CW, Cha BS, Kim KR, et al. Frequent, aggressive behaviors of thyroid microcarcinomas in Korean patients. Endocr J. 2006; 53:627–632.
5. Hartl DM, Travagli JP. The updated American Thyroid Association Guidelines for management of thyroid nodules and differentiated thyroid cancer: a surgical perspective. Thyroid. 2009; 19:1149–1151.
6. Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010; 8:1228–1274.
7. British Thyroid Association. Royal College of Physicians Lodon. Guidelines for the management of thyroid cancer. 2nd ed. London: The Lavenham Press;2007. p. 13–15.
8. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.
9. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004; 28:498–501.
10. Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011; 121:487–491.
11. Boland GW, Lee MJ, Mueller PR, Mayo-Smith W, Dawson SL, Simeone JF. Efficacy of sonographically guided biopsy of thyroid masses and cervical lymph nodes. AJR Am J Roentgenol. 1993; 161:1053–1056.
12. Ying M, Ahuja A, Metreweli C. Diagnostic accuracy of sonographic criteria for evaluation of cervical lymphadenopathy. J Ultrasound Med. 1998; 17:437–445.
13. Rosário PW, de Faria S, Bicalho L, Alves MF, Borges MA, Purisch S, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005; 24:1385–1389.
14. Vassallo P, Wernecke K, Roos N, Peters PE. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology. 1992; 183:215–220.
15. Shah JP. Cervical lymph node metastases--diagnostic, therapeutic, and prognostic implications. Oncology (Williston Park). 1990; 4:61–69. discussion 72, 76.
16. Som PM, Curtin HD, Mancuso AA. An imaging-based classification for the cervical nodes designed as an adjunct to recent clinically based nodal classifications. Arch Otolaryngol Head Neck Surg. 1999; 125:388–396.
17. Snozek CL, Chambers EP, Reading CC, Sebo TJ, Sistrunk JW, Singh RJ, et al. Serum thyroglobulin, high-resolution ultrasound, and lymph node thyroglobulin in diagnosis of differentiated thyroid carcinoma nodal metastases. J Clin Endocrinol Metab. 2007; 92:4278–4281.
18. Uruno T, Miyauchi A, Shimizu K, Tomoda C, Takamura Y, Ito Y, et al. Usefulness of thyroglobulin measurement in fine-needle aspiration biopsy specimens for diagnosing cervical lymph node metastasis in patients with papillary thyroid cancer. World J Surg. 2005; 29:483–485.
19. Grodski S, Brown T, Sidhu S, Gill A, Robinson B, Learoyd D, et al. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery. 2008; 144:1038–1043. discussion 1043.
20. Pazaitou-Panayiotou K, Capezzone M, Pacini F. Clinical features and therapeutic implication of papillary thyroid microcarcinoma. Thyroid. 2007; 17:1085–1092.
21. Mazzaferri EL. Managing small thyroid cancers. JAMA. 2006; 295:2179–2182.
22. Vasileiadis I, Karakostas E, Charitoudis G, Stavrianaki A, Kapetanakis S, Kouraklis G, et al. Papillary thyroid microcarcinoma: clinicopathological characteristics and implications for treatment in 276 patients. Eur J Clin Invest. 2012; 42:657–664.
23. Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994; 18:559–567.
24. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003; 237:399–407.
25. Arora N, Turbendian HK, Kato MA, Moo TA, Zarnegar R, Fahey TJ 3rd. Papillary thyroid carcinoma and microcarcinoma: is there a need to distinguish the two? Thyroid. 2009; 19:473–477.
26. Machens A, Hinze R, Thomusch O, Dralle H. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J Surg. 2002; 26:22–28.
27. Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am. 1996; 5:43–63.
28. Gimm O, Rath FW, Dralle H. Pattern of lymph node metastases in papillary thyroid carcinoma. Br J Surg. 1998; 85:252–254.
29. Page C, Biet A, Boute P, Cuvelier P, Strunski V. 'Aggressive papillary' thyroid microcarcinoma. Eur Arch Otorhinolaryngol. 2009; 266:1959–1963.
30. Joo JY, Park JY, Yoon YH, Choi B, Kim JM, Jo YS, et al. Prediction of occult central lymph node metastasis in papillary thyroid carcinoma by preoperative BRAF analysis using fine-needle aspiration biopsy: a prospective study. J Clin Endocrinol Metab. 2012; 97:3996–4003.
31. Kim BY, Jung CH, Kim JW, Lee SW, Kim CH, Kang SK, et al. Impact of clinicopathologic factors on subclinical central lymph node metastasis in papillary thyroid microcarcinoma. Yonsei Med J. 2012; 53:924–930.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download