Abstract
Meningiomas arising outside the intracranial compartment are known as extradural meningiomas. Extradural meningiomas are rare conditions, accounting for less than 2% of all meningiomas. Primary intraosseous meningioma is used to describe a subset of extradural meningiomas arising from bone. A 46-year-old woman presented with left exophthalmos. Computed tomography and magnetic resonance images revealed an expansile bony lesion in the orbital lateral wall of the left sphenoid bone. The patient underwent craniotomy for excision of the bony lesion. Pathologic examination revealed an intraosseous meningioma.
Meningiomas are the most common benign intracranial neoplasm commonly arising from arachnoid cap cells located in the external layer of the arachnoid membrane. Almost 1% to 2% of meningiomas are lesions described as extradural meningiomas without association with the surface of the arachnoid membrane. Primary intraosseous meningiomas are rare (1, 2). Frontoparietal and orbital regions are the most common sites for intraossseous meningiomas (2). Here, we report a case of primary intraosseous meningioma arising in the orbit.
A 46-year-old woman presented with left exophthalmos. She first noticed it approximately two years ago. She had experienced occasional blurred vision without diplopia or pain. Neurological examination was normal. She had no history of antecedent trauma. Laboratory studies were unremarkable. Orbital magnetic resonance imaging (MRI) revealed an expansible bony lesion arising from the orbital lateral wall of the left sphenoid bone accompanied by dural lesion. This bony lesion showed hypointensity on T1- and T2-weighted images with slightly heterogeneous enhancement after administration of gadolinium. However, the dural lesion was iso- and hyperintense on T1- and T2-weighted images with intense homogeneous enhancement after administration of gadolinium. The adjacent brain parenchyme in the left temporal lobe showed no abnormality (Fig. 1). Precontrast facial computed tomography (CT) images showed abnormal bony expansion with increased sclerosis of the medullary space without visible thickened cortex in the orbital lateral wall of the left sphenoid bone. In addition, there were multiple pin-point low-attenuated foci in the bony lesion. Postcontrast CT images showed markedly enhancing plaques similar to dural thickening adjacent to the bony lesion (Fig. 2).
The patient underwent craniotomy for the excision of the bony lesion in the left lateral orbital bony wall. The mass was composed of bony architecture and vascular structure. Pathological examination indicated the presence of a meningioma. Within the low-power microscopic fields (H&E stain, × 100) (Fig. 3A), multiple cell nests showing infiltration into bone were revealed. Within the high-power microscopic fields (H&E stain, × 400) (Fig. 3B), the neoplastic cells had an indistinct cell border exhibiting whorled architecture. These cells showed round to oval nuclei with even chromatin. A few intranuclear cytoplasmic inclusions were identified. Immunohistochemically, these tumor cells showed strong reactivity to vimentin and epithelial membrane antigen (EMA), supporting their differentiation into meningothelial cells (Fig. 3C). Based on histopathologic findings and immunohistochemical staining results, this tumor was diagnosed as meningioma.
Most meningiomas are considered primary intradural lesions located in the subdural space. In contrast, extradural meningiomas arise from the neck, skin, and nasopharynx. Extracranial meningiomas are rare, accounting for 1-2% of all meningiomas. Primary intraosseous meningiomas are rare form of meningioma. However, they account for approximately 67% of all extradural menigiomas (2). Frontoparietal and orbital regions are the most common sites for intraossseous meningioma. In our case, this tumor was detected at the orbital lateral wall of the left sphenoid bone.
The etiology of intraosseous meningioma has not yet been clarified. Many different hypotheses exist for the origin of primary intraosseous meningioma. This tumor has been thought to arise from ectopic arachnoid cap cells trapped in cranial sutures during molding of the head at birth or from entrapped dura and arachnoid during a trauma with dural tear (3). Although this tumor was closely related to sphenozygomatic suture, only 8% of such tumors are found in the proximity of a cranial suture (3).
Lang et al. (4) have suggested to classify primary extradural meningiomas to type I (purely extracalvarial), type II (purely calvaria,) and type III (calvarial lesions extending beyond the calvaria). Each type is further divided into subgroup of B (skull base) and C (convexity). In our case, the tumor was sphenoidal bony lesion extending beyond the calvaria. Therefore, it could be classified as type III B tumor.
Radiologically, primary intraosseous meningiomas can be classified to osteoblastic, osteolytic, or mixed type. Primary intraosseous meningiomas are mostly osteoblastic type (5). Conventional radiographs can detect abnormalities in 30% to 60% of patients with intraosseous meningiomas, including hyperostosis, atypical vascular marking, and irregular foci of calcification. However, conventional radiographs are usually limited in the diagnosis of extracranial meningiomas due to their superimposed bony structures. CT with bone window showing hyperdense lesions settled in the focal thickened bone is necessary for their detection. After contrast administration, a densely enhanced dural lesion can be found adjacent to the bony lesion. Less frequently, primary intraosseous meningiomas may present as the osteolytic type (6). The lesions appear hypodense on conventional radiographs. The lesions are hyperdense compared to brain tissue on non-enhanced CT scan with homogeneous enhancement after contrast administration. MR images allow better anatomic delineation in the evaluation of soft-tissue component and extradural extension of the lesion. On MR images, the findings of both osteoblastic and osteolytic subtypes of intraosseous meningiomas are similar to each other. Typically, these lesions are hypointense on T1-weighted images and hyperintense on T2-weighted images. After gadolinium administration, homogeneous enhancement can be found. In our case, T2-weighted MR images showed hypointensity accompanying the dural lesion. Thickened and enhanced dura adjacent to neoplasm may represent either reactive inflammation or tumoral invasion. Correctly recognizing dural invasion from reactive change has important surgical implications. Previous study has shown that the presence of pial enhancement, focal dural nodules, or dural thickening of more than 5 mm is highly accurate in predicting the presence of neoplastic dural invasion (7). Because dural thickening in our patient was more than 5 mm, it was more likely a tumor invasion than a reactive dural thickening.
Extradural meningiomas are indistinguishable from intradural meningioma histologically or immunophenotypically. Histopathologic examination often reveals findings pathognomonic of intradural meningiomas, including uniform spindle-shaped cells arranged in whorls, and interconnecting fascicles. Nuclei are oval and regular. Nuclear pseoudoinclusions can be found. Almost all meningiomas should react positively with EMA (stains cytoplasmic membranes) and vimentin (stains cytoplasm) (8).
Differential diagnoses from the osteoblastic type of primary intraossoeus meningioma include fibrous dysplasia, meningioma en plaque, osteoma, osteosarcoma, and Paget disease (8). The osteoblastic type of primary intraosseous meningioma appears as a focal hyperdense skull lesion on CT. Fibrous dysplasia is a developmental disease. It commonly occurs at young age. It stops growing after bone maturation, whereas intraosseous meningioma appears after puberty and continues to grow slowly. In addition, fibrous dysplasia does not have tumor blush on angiography that is typical of meningiomas. Fibrous dysplasia expands the medullary space and widens the bone. An intact rim of cortical bone is often seen over the margins of the involved bone on CT. MR images usually show low to intermediate signals on all imaging sequences with variable enhancement on T1-weighted contrast-enhancing images (9). Malignant transformation is very rare (in less than 1% of cases). Osteomas are the most common benign tumor in calvaria. On CT, osteomas are dense, smooth, and well-demarcated lesions. However, osteomas show void signal without soft-tissue components on MR images. Osteosarcomas usually show poorly defined margins and heterogeneous signals. The intramedullary components of tumors are often associated with the zone of cortical destruction. Imaging features of osteosarcomas are more aggressive. Their prognoses are worse than those of meningiomas. Paget disease of bone shows variable imaging patterns that are related to pathologic stages. Typically, MR images of Paget disease involving carvarium are heterogeneous and hypointense on T1-weighted images due to marrow replacement by fibrous tissue and the presence of nonehancing lesions (10). Serum alkaline phosphatase is typically elevated in patients with Paget disease (2).
Primary intraosseous meningiomas have been observed to be benign and slowly growing. Recent studies indicate that intraosseous meningiomas have a tendency to be more malignant than their intradural counterparts (9). These malignant features may be indicated by microscopic tumor invasion of underlying dura or overlying soft tissue structures. In addition, the potential to transform into malignant is higher in the osteolytic type than in the osteoblastic type (2).
Total tumor removal with wide surgical resection followed by cranial reconstruction is the treatment of choice. When total resection is not feasible because of the involvement of critical structures within the orbit, paranasal sinuses, or skull base, residual tumors should be radiologically followed. Adjuvant therapy must be indicated in patients with malignancy as well as non-resectable tumors. Adjuvant therapies include gamma knife, chemotherapy, and disphosphonate therapy (2, 4).
In conclusion, primary intraosseous meningiomas are rare. They are mostly benign. However, intraosseous meningiomas are more likely to be malignant than their intradural counterparts. Therefore, preoperative radiologic evaluation of the size, location, characteristics, and type of tumors is important. In particular, MR imaging may be valuable in the diagnosis and evaluation of soft tissue component and extradural extension.
Figures and Tables
Fig. 1
A 46-year-old woman presented with left exophthalmos. Axial T1-weighted (A) and T2-weighted (B), and gadolinium-enhanced axial T1-weighted (C) MR images show an expansile bony lesion in the left lateral wall of the orbit. The intraosseous component (white arrow) is hypointense on T1- and T2-weighted images. The soft tissue component (black arrow) that represents underlying dura behind the sphenoid ridge is iso- and hyperintense on T1- and T2-weighted images with intense enhancement after administration of gadolinium.

Fig. 2
CT images of a 46-year-old woman presented with left exophthalmos.
A. Pre-contrast CT scan with bone widow shows an expansile and sclerotic bony lesion (arrow) in the orbital lateral wall of the left sphenoid bone. There are multiple pin-point low-attenuated foci in the bony lesion.
B. Post-contrast CT images show a slightly enhancing plaque-like dural thickening (white arrow) adjacent to the bony lesion (black arrow).
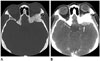
Fig. 3
Histopathological feature of intraosseous hemangioma.
A. Microscopic examination reveals a set of neoplastic cell lobules between bony trabecula (hematoxylin & eosin stain, × 100).
B. Within a high-power field, the tumor cells have indistinct cell borders and show a whorled appearance (hematoxylin & eosin stain, × 400).
C. Immunohistochemical staining for vimentin shows strong cytoplasmic reactivity (× 400).

References
1. Al-Khawaja D, Murali R, Sindler P. Primary calvarial meningioma. J Clin Neurosci. 2007; 14:1235–1239.
2. Elder JB, Atkinson R, Zee CS, Chen TC. Primary intraosseous meningioma. Neurosurg Focus. 2007; 23:E13.
3. Azar-Kia B, Sarwar M, Marc JA, Schechter MM. Intraosseous meningioma. Neuroradiology. 1974; 6:246–253.
4. Lang FF, Macdonald OK, Fuller GN, DeMonte F. Primary extradural meningiomas: a report on nine cases and review of the literature from the era of computerized tomography scanning. J Neurosurg. 2000; 93:940–950.
5. Agrawal V, Ludwig N, Agrawal A, Bulsara KR. Intraosseous intracranial meningioma. AJNR Am J Neuroradiol. 2007; 28:314–315.
6. Arana E, Diaz C, Latorre FF, Menor F, Revert A, Beltrán A, et al. Primary intraosseous meningiomas. Acta Radiol. 1996; 37:937–942.
7. Eisen MD, Yousem DM, Montone KT, Kotapka MJ, Bigelow DC, Bilker WB, et al. Use of preoperative MR to predict dural, perineural, and venous sinus invasion of skull base tumors. AJNR Am J Neuroradiol. 1996; 17:1937–1945.
8. McGuire TP, Palme CE, Perez-Ordonez B, Gilbert RW, Sándor GK. Primary intraosseous meningioma of the calvaria: analysis of the literature and case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 104:e34–e41.
9. Inagaki K, Otsuka F, Matsui T, Ogura T, Makino H. Effect of etidronate on intraosseous meningioma. Endocr J. 2004; 51:389–390.
10. Politi M, Romeike BF, Papanagiotou P, Nabhan A, Struffert T, Feiden W, et al. Intraosseous hemangioma of the skull with dural tail sign: radiologic features with pathologic correlation. AJNR Am J Neuroradiol. 2005; 26:2049–2052.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download