Abstract
Purpose
To evaluate the technical success and complication rates of tunneled-cuffed catheter insertions radiologically placed via the internal jugular vein in patients with different types of underlying diseases.
Materials and Methods
A total of 2153 tunneled-cuffed catheter insertions performed in 1926 patients between January 2008 and December 2012 were retrospectively reviewed. All procedures were conducted using sonography and fluoroscopy. The number of catheter maintenance days, technical success rates, and complication rates were analyzed based on radiologic and medical records.
Results
A total of 204809 catheter maintenance days (mean, 95.35 days; range, 0-1710 days) were recorded. Technical success was achieved in 2148 insertions (99.77%). A total of 185 complications (8.61%, 0.903/1000 catheter days) were observed, including 22 procedure-related complications (1.02%). A total of 143 catheters (6.66%) were removed due to complications. Significant differences in complication rates were observed between patients with or without underlying hematologic diseases (11.65% vs. 7.02%, respectively; p = 0.000). Significant differences in catheter thrombosis were observed between patients in which right-sided or left-sided venous approaches were used (0.81% vs. 2.70%, respectively; p = 0.010).
Vascular access using a central venous catheter has become an essential component of modern medical care (1). The majority of venous access procedures were historically performed by surgeons (2). However, with the benefit of sonographic and fluoroscopic guidance, interventional radiologists have been safety performing the placement of central venous catheters (3). Several studies have reported a high technical success rate and a low complication rate for tunneled-catheter insertion via the internal jugular vein (IJV) using sonographic and fluoroscopic guidance (4, 5, 6, 7, 8). However, to our knowledge, there have been no reports comparing the outcomes of tunneled-cuffed catheter insertions among patients with different underlying diseases, such as kidney or hematologic disorders.
The aim of this study was to evaluate the outcome of tunneled-cuffed catheter insertions placed radiologically via the IJV in patients with different types of underlying disorders.
A total of 2153 tunneled-cuffed catheter insertions were performed via the internal jugular vein in 1926 patients [1087 men and 839 women, including 51 children (range, 4-12 years)] by 3 interventional radiologists between January 2008 and December 2012. The interventional radiologists had 10, 5, and 1 years of experience each. The mean age of the patients was 57.66 years (range, 4-97 years). The demographic data and underlying diseases are shown in Table 1. This retrospective study was approved by the Institutional Review Board at our institution.
The indications for catheter placement were hemodialysis, chemotherapy, plasmapheresis, total parenteral nutrition, and administration of intravenous medications or blood products. Three types of 14.5-Fr tunneled-cuffed catheters were used: HemoSplit, HemoStar (Bard Access Systems, Salt Lake City, UT, USA), Vaxcel Plus Chronic Dialysis Catheter (Boston Scientific, Natick, MA, USA), and MAHURKAR Maxid Dual Lumen Catheters (Covidien, Mansfield, MA, USA). The contraindications of the procedure were as follows: active systemic infection, uncontrolled severe thrombocytopenia or coagulopathy, bilateral IJV occlusion, and superior vena cava (SVC) occlusion.
The blood cell counts, including the white blood cell (WBC) and platelet counts, were obtained in all patients on the day of the procedure to evaluate the correlation between infection and the WBC count, and between thrombotic dysfunction, hemorrhage, or venous thrombosis and the platelet count.
All of the procedures were performed in an interventional radiology suite, and written informed consent was obtained from the patients prior to the procedure. Antibiotic prophylaxis is not used routinely in our institute. If the patient was uncooperative, a sedative (2.5 mg of midazolam initially in adults, up to 5 mg; 0.1 mg/kg of midazolam or 0.01 cc/kg of ketamine in children) was administered intravenously, and the doctor maintained a close watch on the patient. All patients were monitored with a pulse oximeter, electrocardiography, and noninvasive blood pressure cuffs.
An examination of the IJV was performed prior to skin preparation using ultrasound in order to evaluate the vessel size and patency. The right IJV was preferred as the initial access route because it has a straight course and facilitates catheterization (9). The left IJV was chosen in 185 cases because of right mastectomy, right chest wall radiation therapy, venous thrombosis on ultrasound, or the presence of a right-sided catheter.
The access site was initially prepared and draped using standard surgical techniques with betadine solution. All procedures were performed in a routine manner using ultrasound and fluoroscopy with sterile conditions. The catheter tip was located at the right atrium or cavoatrial junction. The patency of the catheter was checked by aspirating blood and injecting heparinized saline without resistance. After disinfecting and dressing the catheters and wounds, a chest radiograph was obtained using fluoroscopy to determine the location of the catheter and the presence of complications, such as catheter kinking or pneumothorax (1).
After the catheter was placed, a diluted heparin solution was injected through each lumen to fill them. Disinfection and dressing of the catheter and exchange of the lock heparin cap were done at the same time every 7-10 days.
Medical records and radiologic images, such as fluoroscopic images, chest radiographs, and computed tomography (CT) scans were reviewed retrospectively. The data were obtained from January 2008 and December 2012. A total of 111 catheters were maintained until December 2012. The remaining catheters were not maintained because the catheter was removed or exchanged (n = 945), lost to follow up (n = 754), or the patients had died (n = 338) (Fig. 1). The last date of follow up was defined as the date of the latest visit to the hospital (written medical record documenting the catheter or the last image study revealing the catheter placement), the death date by a medical record, or the date of catheter removal or exchange. The catheter maintenance days were defined as the number of days between the date of catheter insertion and the last date of follow up.
Technical success was defined as the catheter tip placed precisely at the correct location (at the right atrium or cavoatrial junction) (Fig. 2A), and both lumens functioning well during blood aspiration and the administration of heparinized saline (without resistance) at the end of the procedure.
Complications were divided into 2 groups based on the timing of onset: early [≤ 30 days, including the periprocedural period (≤ 24 hours)] or late (> 30 days), and were also divided into major and minor complications according to the Society of the Interventional Radiology (SIR) guidelines (1). According to the SIR classification, complications requiring no therapy or less than 24 hours of observation are considered minor complications, while those requiring more than 24 hours of hospitalization are considered major complications.
Catheter malfunction was defined as the inability to perform infusion or aspiration with the catheter showing increased resistance during flushing. Moreover, catheter malfunction was divided into catheter thrombosis and non-thrombotic malfunction, including catheter migration and catheter kinking. In this study, catheter thrombosis was referred to as the obstruction of the catheter tip or lumen and was confirmed after catheter removal or thrombolytic therapy. Catheter migration and catheter kinking were confirmed by fluoroscopic images or chest radiographs (Figs. 2B, 3, respectively).
Persistent bleeding was defined as bleeding at the venous puncture site or catheter exit site requiring hemostasis (e.g., suturing of the catheter exit site) or catheter removal. Venous thrombosis was defined as focal or segmental thrombosis in an IJV or SVC on CT scans.
Catheter-related infection (CRI) included local infections (tunnel infection, exit site infection, or wound infection) and catheter-related sepsis. Local infections were defined as the presence of erythema, tenderness, and induration at the catheter exit site, incision wounds, or along the tunnel. Catheter-related sepsis was defined when a blood and catheter tip culture was positive, and the clinical signs and symptoms of sepsis were present without other source of infection (1). Some CRIs were suspected in the clinical setting, despite negative laboratory results.
The rate of catheter complications per 1000 catheter days was calculated as follows: the total number of complications divided by the total number of catheter maintenance days multiplied by 1000. Calculating the rate of complications per 1000 catheter days allowed for standardized comparison to other studies (10).
Statistical analyses were performed using IBM SPSS Statistics software (version 20; IBM, Armonk, NY, USA). The chi-square test, Fisher's exact test, one-way analysis of variance, and logistic regression analysis were used. A p value of < 0.05 was considered statistically significant.
A total of 2153 tunneled-cuffed catheter insertions were attempted in 1926 patients. Technical success was achieved in 2148 insertions (99.77%). The five failures resulted due to left IJV stenosis (n = 2), left brachiocephalic vein stenosis (n = 1), acute angulation between the left IJV and left brachiocephalic vein (n = 1), and catheter kinking near the puncture site (n = 1). All of these failures occurred during the attempt to catheterize the left IJV. However, all of these 5 cases underwent successful catheter placement on the following day. A total of 204809 catheter maintenance days (mean, 95.35 days; range, 0-1710 days) were recorded. The right IJV and left IJV were used in 1968 cases (91.41%) and 185 cases (8.59%), respectively.
A total of 945 catheters (44%) were removed or exchanged. Among these, 802 catheters (37.33%) were removed because of the completion of treatment or a change in the treatment plan, including conversion to an arteriovenous shunt or continuous ambulatory peritoneal dialysis. A total of 143 catheters (6.66%) were removed or exchanged because of complications, including CRI (n = 117), venous thrombosis (n = 7), catheter thrombosis (n = 7), catheter migration (n = 6), persistent bleeding (n = 4), and catheter kinking (n = 2).
A total of 185 complications (0.903/1000 catheter days) were observed. There were 81 early catheter complications (0.395/1000 catheter days) and 104 late complications (0.508/1000 catheter days) (Table 2). In addition, 4 periprocedural complications (≤ 24 hours, 0.19%) occurred in renal failure patients, including 3 persistent bleedings and 1 catheter kinking. There was no periprocedural complications, such as pneumothorax or air embolism.
There were 124 major complications (0.605/1000 catheter days), such as CRI and venous thrombosis, and 61 minor complications (0.298/1000 catheter days), including persistent bleeding, catheter thrombosis, catheter migration, and catheter kinking. CRIs occurred in 117 insertions (5.45%, 0.571/1000 catheter days) and tended to develop later (mean, 113.26; range, 4-547 days). All catheters were removed immediately. A total of 18 CRIs (0.84%) occurred within 14 days after the procedure, with 12 and 6 cases involving renal failure and hematologic diseases, respectively.
Persistent bleeding occurred in 25 insertions (1.16%, 0.122/1000 catheter days) and developed early in all cases (mean, 3.00; range, 1-6 days). These were handled by catheter removal (n = 4) and additional suturing of the incision sites (n = 21). Venous thrombosis occurred in 7 insertions (0.33%, 0.034/1000 catheter days) and tended to develop later (mean, 137.00; range, 11-267 days). Venous thrombosis involved the right IJV (n = 4), SVC (n = 2), and left brachiocephalic vein to the SVC (n = 1). All of the catheters had to be removed. Catheter thrombosis was recorded for 21 insertions (0.98%, 0.103/1000 catheter days) and also tended to develop later (mean, 110.48; range, 3-470 days). These were handled by catheter exchange (n = 13), catheter removal (n = 7), and the administration of urokinase (n = 1).
Catheter migration occurred in 10 insertions (0.47%, 0.049/1000 catheter days; mean, 58.22; range, 3-178 days). These were handled by catheter reposition (n = 2), catheter exchange (n = 2), and catheter removal (n = 6). Catheter kinking occurred in 5 insertions (0.23%, 0.024/1000 catheter days) and was an early complication (mean, 12.40; range, 1-27 days). These were handled by catheter reposition (n = 2), catheter exchange (n = 1), and catheter removal (n = 2). Three of these catheters were kinked on the initial chest radiograph after catheter insertion.
Of the 185 complications, 86 (11.65%) occurred in patients with hematologic disease; 96 (7.17%), in patients in renal failure; and 3 (5.08%), in patients with solid tumors (Table 3). No complications were observed in the remaining patients. Many of the complications occurred due to infections in the hematologic disease group (71/86) and the renal failure group (43/96). All 7 cases of venous thrombosis were observed in the former group. Additionally, the majority of hemorrhages (24/25) and all 5 cases of catheter kinking occurred in the latter group. The solid tumor group had only 3 CRIs.
Statistically significant differences were observed in the complication rates between patients with hematologic or non-hematologic diseases (11.65% vs. 7.03%, p = 0.000) (Table 4). Major complications occurred more frequently in the hematologic disease groups, and minor complications occurred more frequently in the non-hematologic disease groups (p = 0.000). Similarly, the infectious complication rates and incidence of hemorrhaging differed significantly between these 2 groups (9.61% vs. 3.26%, p = 0.000; 0.13% vs. 1.79%, p = 0.000, respectively) (Table 3). However, no significant relationship was found between hemorrhaging and the platelet count in the renal failure group (p = 0.012, odds ratio: 0.993).
A total of 1968 catheter insertions were achieved via the right IJV, whereas 180 catheter insertions were achieved via the left IJV. No significant differences were observed between the access routes and the overall complication rates (p = 0.487) (Table 5). However, significant differences were found between the access routes and thrombotic dysfunction (right, 0.81%; left, 2.70%; p = 0.029).
The mean platelet and WBC counts at the time of catheter insertion were 1678200/mm3 and 11900/mm3, respectively. The blood cell counts according to the underlying diseases are summarized in Table 6. Significant differences in the platelet counts but not the WBC counts were found between patients with different underlying diseases (p = 0.000 and p = 0.298, respectively).
Tables 7 and 8 summarize the platelet and WBC counts according to the complications and underlying diseases. Significant relationships were observed between persistent bleeding and the platelet count in the renal failure group (mean, 132.29 × 103/mm3, p = 0.012) but not between CRI and the WBC count.
In the present study, we successfully inserted 2148 catheters and achieved a technical success rate of 99.77%. A total of 185 complications occurred, resulting in a complication rate of 8.61%. However, we achieved an extremely low incidence of procedure-related complications. Procedure-related complications are more likely to occur within 24 hours of the procedure and are usually associated with catheter malposition and injury of surrounding structures (1, 11). In the present study, only 4 periprocedural complications were observed; these all occurred in the renal failure patients and included persistent bleeding and catheter kinking. Moreover, only 18 CRIs occurred within 14 days in the study population. CRIs occurring at more than 14 days after insertion have little relationship with the catheter insertion technique and are a result of poor catheter management (12). In adopting the procedure at our institute, we believe procedure-related infectious complications might be lower through maintaining sterile conditions. The extremely low incidence of procedure-related complications is in agreement with this focused approach on maintaining sterile conditions.
All 5 procedural failures occurred during attempts to catheterize the left IJV. According to a randomized prospective study (13), a higher incidence of failure is associated with left, as compared to right, internal jugular catheterization. Hence, our study findings are consistent with this previous report. Moreover, 3 of the failed cases also had venous steno-occlusive diseases (2 cases, IJV stenoses; 1, brachiocephalic vein stenosis). According to Rose et al. (14), insertion failures in patients with this condition might be avoided by using pulsed Doppler sonography.
Moureau et al. (10) performed the largest study on complications resulting from insertion of central venous catheters, reporting 767 complications in 8406 catheters (9.12%, 1.01/1000 catheter days). The present complication rate of 8.61% and 0.903 per 1000 catheters reflects similar, but slightly better, results. Moreover, this previous study (12) reported 534 infections in 8406 catheters (6.35%, 0.70/1000 catheter days), while Nightingale et al. (15) observed 47 infections in 817 catheters (5.8%, 0.47/1000 catheter days). Compared to these previous studies, we achieved a slightly lower infectious complication rate (5.45%, 0.571/1000 catheter days). Furthermore, a previous prospective study reported 11.5% (0.09/100 catheter days) of infectious complications occurred in hematologic patients with tunneled central venous catheters (16). The present results were similar to those of this previous report but were slightly lower (9.61%, 71 cases in 738 insertions).
In the present study, the CRI rates differed significantly between patients with hematologic diseases and those with other diseases. However, no significant differences in the incidence of CRIs were found between the underlying diseases and WBC counts in CRI. Most patients with hematologic diseases had leukemia and lymphoma, and higher WBCs were present in these patients than in those with other diseases. A possible explanation for the increased infection rate is the WBCs in these patients were malfunctioning abnormal cells, resulting in reduced immunity in these patients as compared to those with different underlying diseases.
Persistent bleeding was the second most common complication in our study. In total, 25 cases of persistent bleeding required hemostasis or catheter removal. Of these, 24 and 1 occurred in the renal failure and hematologic groups, respectively, and the incidence of this complication significantly differed between them. Importantly, a significant relationship was found between platelet count and hemorrhaging in the renal failure group. Paradoxically, the platelet count in the renal failure group was more than that in the hematologic group. Because most of the hematologic patients underwent catheter placement routinely for chemotherapy, these patients may have been corrected for abnormal conditions before the procedure. However, one third of the renal failure patients had acute kidney injuries, an urgent condition, which may have resulted in undergoing catheter placement before the correction of abnormal conditions. Moreover, many of the renal failure patients had other underlying systemic diseases (e.g., diabetes). As is well known, acute and chronic renal failure patients have bleeding diathesis, which is considered to be a multifactorial condition (17). Many authors consider hemorrhage in renal failure patients to be an inevitable result of poor platelet function rather than a complication of the procedure (18). Obialo et al. (19) reported a high incidence, 42%, for the hemorrhagic complication rate in renal failure patients. Based on these previous and present findings, hemorrhagic complications might occur more frequently in renal failure patients than in hematologic disease patients.
Central venous catheter-related venous thrombosis occurred only in the hematologic patients and was observed at a relatively high frequency in this group. Six of the 7 patients with this complication had symptoms such as facial, neck, or arm swelling. The remaining patient had no symptoms, as the venous thrombosis was found incidentally by a follow-up chest CT. Several previous studies have reported central venous catheter-related thrombosis occurring at an incidence of 1.2-34.1% in hematologic patients (20, 21, 22, 23). Our study results were similar to these previous findings. Tesselaar et al. (24) reported the risk of venous thrombosis was 3.5-fold higher for left-sided compared to right-sided placement. However, in our study, no significant relationship was found between the left- and right-sided placements.
Catheter malfunction (e.g., catheter thrombosis) occurred in the patients with hematologic disease and renal failure. Catheter thrombosis occurred in 0.98% of the patients (0.103/1000 catheter days). Caridi et al. (7) reported a catheter thrombosis rate of 6.7% (0.16/100 catheter days). Hence, the incidence of catheter thrombosis in the present study is lower than that in the previous one. Significant differences were found between the right and left venous approaches, but the basis of this difference remains unclear.
Wong et al. (25) previously reported an incidence of 0.6% for catheter kinking and found it frequently occurred in the very thin patients. In our study, 3 of the 5 patients with catheter kinking were slim (body mass index of -16). An immediate chest radiograph after the procedure revealed catheter kinking in these 3 patients. The same operator performed the catheter insertion in these 3 patients and was relatively inexperienced in this procedure. Hence, the occurrence of this complication might also depend on the operator's experience. In terms of catheter migration, Moureau et al. (10) previously reported 63 catheters were pulled out from 8406 total catheters (0.75%), while Nightingale et al. (15) observed 3.5% of the catheters migrated (0.29/1000 catheter days). In our study, catheter migration occurred in 10 insertions (0.47%, 0.049/1000 catheter days). Hence, our findings are consistent with these previous findings.
The blood cell counts were obtained for all patients on the day of the procedure. Hence, a modest relationship between the blood cell count and complication rate is possible, since the blood cell count reflected the patient status on the day of the procedure and not the one when the complication developed. Further, all patients with thrombocytopenia on the day of the procedure received 5-10 packs of platelet concentrate before the procedure. However, the platelet counts did not reflect the transfused platelet concentrate. Hence, further study will be needed to address the relationship between platelet count and incidence of complications.
This study had several limitations. First, we retrospectively reviewed the medical records, including physicians' progress notes and nurses' notes; however, some data might have been omitted from these medical records. Moreover, because of the lack of medical records, we were unable to evaluate periprocedural complications. Second, the solid tumor group and the one designated "others" had significantly fewer patients than the hematologic and renal failure groups. Similarly, the left IJV approach involved fewer cases than the right. Third, patients were categorized according to the causative underlying disease for catheter placement. For example, if a patient who underwent catheter placement for hemodialysis had end-stage renal disease and rectal cancer, this patient was categorized in the end-stage renal group. Moreover, we did not consider variables (e.g., diabetes) that could have influenced the results. Fourth, because the WBC count, rather than neutrophil count, was obtained, a limitation might be present in the analysis of the relationship of blood cell count to incidence of CRIs. Fifth, some catheters were removed without positive laboratory results if patients were suspected of having CRIs (e.g., unexplained fever), since many patients were immunocompromised. Thus, we might have included infectious complications not associated with the catheter. Finally, asymptomatic thrombosis might have occurred but been undetected because routine image studies for venous thrombosis were not performed during follow-up. In particular, venography via an indwelling catheter in cases of catheter malfunction was not routinely performed; therefore, complication rates of venous or catheter thrombosis might be underestimated.
In summary, a very high technical success rate and very low procedure-related complication rate were achieved. Hematologic patients had a somewhat higher complication rate than other patients, and infection represented the most frequent and challenging complication in the study population. Particular attention should be focused on hematological patients to reduce complications. In conclusion, the tunneled-cuffed catheter insertion placed radiologically via IJV is safe and effective in patients with various underlying diseases.
Figures and Tables
Fig. 2
Radiographs of a 62-year-old man who had an acute kidney injury.
A. Chest anteroposterior radiograph obtained after tunneled catheter placement shows the tip of the catheter located at the cavoatrial junction (arrow).
B. Chest posteroanterior radiograph obtained 6 months after catheter placement. The catheter is migrated and its tip is located at the clavicle level (arrow). The catheter had been removed on that day, and re-inserted on the following day.
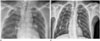
Fig. 3
Chest posteroanterior radiograph of a 50-year-old woman who had an end-stage renal disease. It shows a kink in a malfunctioning tunneled catheter (arrow).
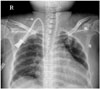
Table 1
Study Population

Table 2
Complications Expressed Relative to Catheter Days

Table 3
Complications According to Underlying Diseases

Table 4
Major or Minor Complications According to Hematologic or Non-Hematologic Underlying Diseases

| Major* | Minor† | Total | |
|---|---|---|---|
| Hematologic disease | 78 (10.57%) | 8 (1.08%) | 86 (11.65%) |
| Non-hematologic disease | 46 (3.26%) | 53 (3.76%) | 99 (7.02%) |
Table 5
Comparison of Complication Rates Based on Access Routes

Table 6
Blood Cell Counts According to Underlying Diseases

Table 7
Mean Platelet Count for Complications of Different Underlying Diseases

References
1. Silberzweig JE, Sacks D, Khorsandi AS, Bakal CW. Society of Interventional Radiology Technology Assessment Committee. Reporting standards for central venous access. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S443–S452.
2. Reeves AR, Seshadri R, Trerotola SO. Recent trends in central venous catheter placement: a comparison of interventional radiology with other specialties. J Vasc Interv Radiol. 2001; 12:1211–1214.
3. Funaki B. Tunneled central venous catheter insertion. Semin Intervent Radiol. 2008; 25:432–436.
4. Cavanna L, Civardi G, Vallisa D, Di Nunzio C, Cappucciati L, Bertè R, et al. Ultrasound-guided central venous catheterization in cancer patients improves the success rate of cannulation and reduces mechanical complications: a prospective observational study of 1,978 consecutive catheteri-zations. World J Surg Oncol. 2010; 8:91.
5. Peynircioglu B, Ozkan F, Canyigit M, Pamuk GA, Geyik S, Cil BE, et al. Radiologically placed tunneled internal jugular catheters in the management of chronic hemodialysis and long-term infusion therapies in the pediatric population. J Vasc Interv Radiol. 2007; 18:875–881.
6. Trerotola SO, Johnson MS, Harris VJ, Shah H, Ambrosius WT, McKusky MA, et al. Outcome of tunneled hemodialysis catheters placed via the right internal jugular vein by interventional radiologists. Radiology. 1997; 203:489–495.
7. Caridi JG, Grundy LS, Ross EA, Prabhu PN, Tonkin JC, Hawkins IF Jr, et al. Interventional radiology placement of twin Tesio catheters for dialysis access: review of 75 patients. J Vasc Interv Radiol. 1999; 10:78–83.
8. Koroglu M, Demir M, Koroglu BK, Sezer MT, Akhan O, Yildiz H, et al. Percutaneous placement of central venous catheters: comparing the anatomical landmark method with the radiologically guided technique for central venous catheterization through the internal jugular vein in emergent hemodialysis patients. Acta Radiol. 2006; 47:43–47.
9. Funaki B. Central venous access: a primer for the diagnostic radiologist. AJR Am J Roentgenol. 2002; 179:309–318.
10. Moureau N, Poole S, Murdock MA, Gray SM, Semba CP. Central venous catheters in home infusion care: outcomes analysis in 50,470 patients. J Vasc Interv Radiol. 2002; 13:1009–1016.
11. Dariushnia SR, Wallace MJ, Siddiqi NH, Towbin RB, Wojak JC, Kundu S, et al. Quality improvement guidelines for central venous access. J Vasc Interv Radiol. 2010; 21:976–981.
12. Mauro MA, Jaques PF. Insertion of long-term hemodialysis catheters by interventional radiologists: the trend continues. Radiology. 1996; 198:316–317.
13. Sulek CA, Blas ML, Lobato EB. A randomized study of left versus right internal jugular vein cannulation in adults. J Clin Anesth. 2000; 12:142–145.
14. Rose SC, Kinney TB, Bundens WP, Valji K, Roberts AC. Importance of Doppler analysis of transmitted atrial waveforms prior to placement of central venous access catheters. J Vasc Interv Radiol. 1998; 9:927–934.
15. Nightingale CE, Norman A, Cunningham D, Young J, Webb A, Filshie J. A prospective analysis of 949 long-term central venous access catheters for ambulatory chemotherapy in patients with gastrointestinal malignancy. Eur J Cancer. 1997; 33:398–403.
16. Lee SH, Hahn ST. Comparison of complications between transjugular and axillosubclavian approach for placement of tunneled, central venous catheters in patients with hematological malignancy: a prospective study. Eur Radiol. 2005; 15:1100–1104.
17. Galbusera M, Remuzzi G, Boccardo P. Treatment of bleeding in dialysis patients. Semin Dial. 2009; 22:279–286.
18. Kerr A, Pathalapati R, Qiuhu S, Baumstein D. Purse-string suture to prevent bleeding after tunneled dialysis catheter insertion. J Vasc Interv Radiol. 2008; 19:1176–1179.
19. Obialo CI, Conner AC, Lebon LF. Tunneled hemodialysis catheter survival: comparison of radiologic and surgical implantation. ASAIO J. 2000; 46:771–774.
20. Male C, Chait P, Andrew M, Hanna K, Julian J, Mitchell L, et al. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood. 2003; 101:4273–4278.
21. Magagnoli M, Masci G, Castagna L, Pedicini V, Poretti D, Morenghi E, et al. Prophylaxis of central venous catheter-related thrombosis with minidose warfarin in patients treated with high-dose chemotherapy and peripheral-blood stem-cell transplantation: retrospective analysis of 228 cancer patients. Am J Hematol. 2006; 81:1–4.
22. van Rooden CJ, Rosendaal FR, Barge RM, van Oostayen JA, van der Meer FJ, Meinders AE, et al. Central venous catheter related thrombosis in haematology patients and prediction of risk by screening with Doppler-ultrasound. Br J Haematol. 2003; 123:507–512.
23. Fijnheer R, Paijmans B, Verdonck LF, Nieuwenhuis HK, Roest M, Dekker AW. Factor V Leiden in central venous catheter-associated thrombosis. Br J Haematol. 2002; 118:267–270.
24. Tesselaar ME, Ouwerkerk J, Nooy MA, Rosendaal FR, Osanto S. Risk factors for catheter-related thrombosis in cancer patients. Eur J Cancer. 2004; 40:2253–2259.
25. Wong JK, Sadler DJ, McCarthy M, Saliken JC, So CB, Gray RR. Analysis of early failure of tunneled hemodialysis catheters. AJR Am J Roentgenol. 2002; 179:357–363.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download