Abstract
Krukenberg tumors recognized during pregnancy are rarely reported. The preoperative diagnosis can be challenging because of the confusing morphological features and symptoms during pregnancy. Here, we report a case of a 29-year-old pregnant woman at 29 weeks gestation presenting with bilateral solid ovarian masses, which were later diagnosed as metastatic ovarian cancer originating from advanced gastric cancer. This case suggests that Krukenberg tumors should be considered when bilateral ovarian solid masses are encountered regardless of pregnancy.
The incidence of adnexal tumors during pregnancy ranges from 1 in 600-2500 to 1-2% (1). Previous data indicate that most adnexal masses requiring removal during pregnancy are benign (2). However, 2-3% of masses removed during pregnancy are malignant (2). According to a large retrospective review by Leiserowitz et al. (3), 0.93% of adnexal masses associated with pregnancy are cancers. Krukenberg tumors, which are metastatic ovarian tumors originating from the gastrointestinal tract, occurs very rarely during pregnancy at a rate of approximately 1 in 100000 (4). Here, we present a young woman at a 29-week pregnancy who was admitted for ascites detected during prenatal surveillance and revealed to have bilateral Krukenberg tumors originating from advanced gastric cancer.
A 29-year-old multiparous woman in a 29-week pregnancy presented with a large volume of ascites and abnormal liver function test results detected during routine prenatal surveillance. Although she had a history of thrombocytopenia, her platelet count was within the normal range (432000/mL) but aspartate aminotransferase/alanine transaminase/alkaline phosphatase (184/222/200 IU/L) and carbohydrate antigen (CA) 125 was elevated (229.6 U/mL). An ascites analysis indicated a non-hepatic origin (serum-ascites albumin gradient, 0.4).
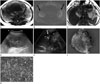
After draining 1.5-2 L of fluid and under the suspicion of pre-eclampsia or ovarian cancer, magnetic resonance imaging (MRI, 3.0 T Skyra; Siemens, Erfurt, Germany) was performed. The MRI sequences were T2-weighted half-Fourier acquisition single shot turbo spin echo (HASTE) axial and coronal images, T1-weighted dual gradient-echo in-phase and opposed-phase chemical shift images and echo planar diffusion-weighted images with b-values of 0, 400, and 800 and an apparent diffusion coefficient map. Bilateral high signal intensity (SI) ovarian solid masses were found on T2-weighted image (T2WI) with diffusion restriction (Fig. 1A, B). Both ovarian masses had smooth margins and several lower SI follicle-like nodules were located in the periphery within the left ovarian mass on a T2W HASTE image. Omental fluffy soft tissues were found along with a large volume of ascites. Irregular gastric wall thickening in the gastric body and antrum were initially neglected due to so-called standing-wave effects in the upper abdomen and the evaluation was inadvertently focused on the ovarian masses (Fig. 1C). Preoperative ultrasound (US) revealed heterogeneously echogenic solid masses with peripheral vessels leading to bilateral ovarian masses raising the suspicion of malignancy (Fig. 1D, E). A cesarean section was performed, and the irregular shaped ovarian masses with a nodular bumpy surface were resected. The baby was male with a body weight of 1500 g. An ascites sample was sent for cytological examination and omental tissue was excised with the ovarian masses, although no gross peritoneal seeding was found. The gross specimen revealed a pinkish to tan surface with multiple nodules, and the cut surface was gelatinous to red and fleshy in appearance mixed with hemorrhage and microcysts (Fig. 1F). Many signet ring cells revealing eosinophilic or grayish cytoplasm with mucin scattered in the ovarian stroma were detected microscopically (Fig. 1G).
Melena was detected during the preoperative evaluation, and postoperative endoscopy revealed large gastric ulcers in the gastric antrum with blood clots, which was confirmed to be poorly differentiated adenocarcinoma with a dominant signet ring cell component. The patient is scheduled to receive chemotherapy for advanced stomach cancer.
Most adnexal masses found during pregnancy are corpus luteal cysts, endometrioid cysts, or mature cystic teratomas (5). There seems to be an extremely low incidence of ovarian malignancy during pregnancy and the malignancies tend to be earlier stages with a less aggressive histology (1, 2). However, about 6% of malignant ovarian tumors are metastatic and Krukenberg tumors can be encountered in young patients more often than other metastatic ovarian neoplasms (4). Unfortunately, the preoperative diagnosis of a Krukenberg tumor secondary to gastric cancer occurring during pregnancy is challenging because pregnancy-related gastric symptoms and morphological features can mask the presence of gastric cancer and ascites. US is the best diagnostic tool to reveal adnexal masses in pregnant and non-pregnant women (5). The sonographic characteristics that have been associated with an increased risk of malignancy are large size (> 7 cm), solid components or complex appearance, papillary projections, internal thick or vascular septations, bilaterality, irregular borders, increased vascularity, low-resistance blood flow, and ascites (2). In our case, US showed bilateral heterogeneously echogenic solid masses, representing features suspicious for malignancy. In addition, a color Doppler image showed peripheral vessels leading to the mass, which were characteristic of the "leading vessel" of metastatic ovarian cancer (6, 7). A high serum CA-125 level is a normal finding during the first trimester, which returns to the normal range (5). The elevated CA-125 level in our patient in the third trimester of pregnancy was another clue for the ovarian abnormality.
The differential diagnosis of a solid ovarian mass detected during pregnancy includes germ cell tumors, epithelial ovarian cancers, sex-cord stromal tumors, or metastasis including Krukenberg tumors (8). Germ cell tumors can be distinguished by their characteristic imaging features (i.e., fat component), and epithelial ovarian cancers may appear as a cystic solid mass rather than a pure solid mass. Sex-cord stromal tumors were considered initially in our case; however, the US findings and bilaterality were clues for metastatic ovarian cancers.
Some studies have reported Krukenberg tumors in 20-31-year-old pregnant women (9, 10). Genç et al. (9) reported a patient with a Krukenberg tumor and a fair prognosis who was disease free 18 months after surgery. However, the prognosis of a Krukenberg tumor remains poor, though early detection and complete resection followed by adjuvant chemotherapy improves survival (10). However, the prognosis worsens when the primary tumor is identified after metastasis to the ovary (4).
In summary, we report a rare case of bilateral Krukenberg tumors in a young pregnant woman with advanced gastric cancer, which was difficult to diagnose preoperatively because of the pregnancy. A Krukenberg tumor should be considered when bilateral solid ovarian masses are encountered, regardless of pregnancy in a young woman, for a timely diagnosis and management planning.
Figures and Tables
Fig. 1
29-year-old woman in a 29-week gestation with bilateral Krukenberg tumors and advanced gastric cancer.
A. T2-weighted half-Fourier acquisition single shot turbo spin echo axial image shows iso to high signal intensity (SI) solid masses in bilateral ovaries with lower SI follicle-like nodules in periphery of the mass (black arrows). Large amount of ascites is present. She is in third trimester pregnancy and fetus in the uterus is seen in this image.
B. Diffusion-weighted axial image (b-value = 800) shows diffusion restriction in bilateral ovarian masses (black arrows).
C. Irregular gastric wall thickening along the gastric body and antrum has been detected retrospectively (black arrows). Omental fluffy soft tissues are also noted (dashed arrow), which is confirmed to be advanced gastric cancer and peritoneal carcinomatosis along with large amount of ascites.
D. Ultrasonography reveals heterogeneously echogenic solid masses in bilateral ovaries (8.6 cm mass in left ovary, right ovary mass is not shown here), raise the suspicion of malignancy.
E. Color Doppler image shows peripheral vascularity leading to right ovarian mass, which is thought to be leading vessel (white arrow).
F. Gross specimen reveals pinkish tan to fleshy appearance with multiple nodularity of the ovarian mass. Hemorrhage and microcysts are also found.
G. In microscopic exam (hematoxylin and eosin stain, × 400) of ovarian mass, many signet ring cells with eosinophilic cytoplasm and abundant mucin (white arrows) are interspersed in the ovarian stroma.
References
1. Machado F, Vegas C, Leon J, Perez A, Sanchez R, Parrilla JJ, et al. Ovarian cancer during pregnancy: analysis of 15 cases. Gynecol Oncol. 2007; 105:446–450.
2. Schwartz N, Timor-Tritsch IE, Wang E. Adnexal masses in pregnancy. Clin Obstet Gynecol. 2009; 52:570–585.
3. Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006; 101:315–321.
4. Papantoniou N, Belitsos P, Hatzipapas I, Rodolakis A, Papaspyrou I, Antsaklis A. Excessive hirsutism in pregnancy because of Krukenberg tumor. J Matern Fetal Neonatal Med. 2012; 25:869–871.
5. Grigoriadis C, Eleftheriades M, Panoskaltsis T, Bacanu AM, Vitoratos N, Kondi-Pafiti A, et al. Ovarian cancer diagnosed during pregnancy: clinicopathological characteristics and management. G Chir. 2014; 35:69–72.
6. Testa AC, Licameli A, Di Legge A, Mascilini F, Petruzziello L, Pelagalli M, et al. Color Doppler sonographic features of a Krukenberg tumor in pregnancy. J Ultrasound Med. 2009; 28:695–698.
7. Testa AC, Mancari R, Di Legge A, Mascilini F, Salutari V, Scambia G, et al. The 'lead vessel': a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008; 31:218–221.
8. Cheng CY, Chen TY, Lin CK, Tsao SM, Shih IF, Shy SW. Krukenberg tumor in pregnancy with delivery of a normal baby: a case report. Zhonghua Yi Xue Za Zhi (Taipei). 1994; 54:424–427.
9. Genç M, Genç B, Solak A, Gür E, Sezgin C. Bilateral Krukenberg tumor in a 16-week pregnant woman. Eur J Gynaecol Oncol. 2014; 35:95–96.
10. Singhal SR, Nanda S, Chaudhry P, Sen J, Singhal SK. Metastatic bilateral malignant ovarian tumors associated with pregnancy. Taiwan J Obstet Gynecol. 2009; 48:167–168.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download