Abstract
Purpose
To compare the diagnostic performance of multidetector computed tomography (MDCT) and magnetic resonance imaging (MRI) in characterization of pancreatic cystic lesions.
Materials and Methods
We conducted a retrospective study on 34 patients with histopathologically proven cystic pancreatic lesions who underwent both preoperative MDCT and MRI. CT and MRI were independently evaluated for differentiating mucinous vs. non-mucinous lesions, differentiating aggressive vs. non-aggressive lesion, analyzing morphological features, and evaluating specific leading diagnoses. Sensitivity, specificity, and accuracy were determined. Competency assessment of lesional morphology analysis was performed using the kappa values of the 2 tests.
Results
The sensitivity, specificity, and accuracy of MRI for differentiating mucinous vs. non-mucinous lesions were higher than CT (p = 0.03). For differentiating aggressiveness, the sensitivity of MRI was better than CT, but the specificity of CT was better than MRI. In evaluation of morphologic features, MRI showed better performance in characterization of septa and wall. Otherwise, the 2 modalities showed similarly good performance. MRI was better than CT in determining a specific diagnosis (58.8% vs. 47.2%, respectively).
Figures and Tables
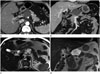 | Fig. 1A 59-year-old male with serous cystadenoma in body of pancreas.
A, B. Axial and coronal reformatted contrast enhanced multidetector CT image reveals unilocular cystic lesion (arrow) with no definite septations. This lesion was misdiagnosed as pseudocyst.
C, D. Axial and coronal T2-weighted MR image reveals multilocular cystic lesion (arrow) with obvious intralesional septa.
|
Table 1
Distribution of Pancreatic Cystic Lesions According to Histopathologic Type and Biological Aggressiveness

Table 2
Validities of Image-Based Diagnosis of CT and MRI for the Diagnosis of Cystic Pancreatic Lesions

References
1. Kirkpatrick ID, Desser TS, Nino-Murcia M, Jeffrey RB. Small cystic lesions of the pancreas: clinical significance and findings at follow-up. Abdom Imaging. 2007; 32:119–125.
2. Handrich SJ, Hough DM, Fletcher JG, Sarr MG. The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol. 2005; 184:20–23.
3. Goh BK, Tan YM, Cheow PC, Chung YF, Chow PK, Wong WK, et al. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006; 192:148–154.
4. Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999; 23:906–912.
5. Sainani NI, Saokar A, Deshpande V, Fernández-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009; 193:722–731.
6. Allen PJ, Jaques DP, D'Angelica M, Bowne WB, Conlon KC, Brennan MF. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003; 7:970–977.
7. Sarr MG, Murr M, Smyrk TC, Yeo CJ, Fernandez-del-Castillo C, Hawes RH, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg. 2003; 7:417–428.
8. Oh HC, Kim MH, Hwang CY, Lee TY, Lee SS, Seo DW, et al. Cystic lesions of the pancreas: challenging issues in clinical practice. Am J Gastroenterol. 2008; 103:229–239. quiz 228, 240.
9. Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003; 138:427–433. discussion 433-434.
10. Megibow AJ, Lombardo FP, Guarise A, Carbognin G, Scholes J, Rofsky NM, et al. Cystic pancreatic masses: cross-sectional imaging observations and serial follow-up. Abdom Imaging. 2001; 26:640–647.
11. Walsh RM, Vogt DP, Henderson JM, Zuccaro G, Vargo J, Dumot J, et al. Natural history of indeterminate pancreatic cysts. Surgery. 2005; 138:665–670. discussion 670-671.
12. Macari M, Megibow AJ. Focal cystic pancreatic lesions: variability in radiologists' recommendations for follow-up imaging. Radiology. 2011; 259:20–23.
13. Balci NC, Semelka RC. Radiologic features of cystic, endocrine and other pancreatic neoplasms. Eur J Radiol. 2001; 38:113–119.
14. Irie H, Honda H, Aibe H, Kuroiwa T, Yoshimitsu K, Shinozaki K, et al. MR cholangiopancreatographic differentiation of benign and malignant intraductal mucin-producing tumors of the pancreas. AJR Am J Roentgenol. 2000; 174:1403–1408.
15. Taouli B, Vilgrain V, O'Toole D, Vullierme MP, Terris B, Menu Y. Intraductal papillary mucinous tumors of the pancreas: features with multimodality imaging. J Comput Assist Tomogr. 2002; 26:223–231.
16. Mergo PJ, Helmberger TK, Buetow PC, Helmberger RC, Ros PR. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics. 1997; 17:281–301.
17. Minami M, Itai Y, Ohtomo K, Yoshida H, Yoshikawa K, Iio M. Cystic neoplasms of the pancreas: comparison of MR imaging with CT. Radiology. 1989; 171:53–56.
18. Katz DS, Friedel DM, Kho D, Georgiou N, Hines JJ. Relative accuracy of CT and MRI for characterization of cystic pancreatic masses. AJR Am J Roentgenol. 2007; 189:657–661.
19. Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR Am J Roentgenol. 2007; 189:648–656.
20. Sahani DV, Sainani NI, Blake MA, Crippa S, Mino-Kenudson M, del-Castillo CF. Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR Am J Roentgenol. 2011; 197:W53–W61.
21. Choi BS, Kim TK, Kim AY, Kim KW, Park SW, Kim PN, et al. Differential diagnosis of benign and malignant intraductal papillary mucinous tumors of the pancreas: MR cholangiopancreatography and MR angiography. Korean J Radiol. 2003; 4:157–162.
22. Kawamoto S, Lawler LP, Horton KM, Eng J, Hruban RH, Fishman EK. MDCT of intraductal papillary mucinous neoplasm of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006; 186:687–695.
23. Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003; 90:1244–1249.
24. Wiesenauer CA, Schmidt CM, Cummings OW, Yiannoutsos CT, Howard TJ, Wiebke EA, et al. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003; 138:610–617. discussion 617-618.
25. Cicchetti DV, Feinstein AR. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol. 1990; 43:551–558.
26. Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol. 1990; 43:543–549.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download