Abstract
Purpose
To compare the effectiveness of portal vein embolization (PVE) performed using gelfoam or a gelfoam-coil combination before major hepatic resection in patients with chronic liver disease.
Materials and Methods
PVE using gelfoam or a gelfoam-coil combination was performed in 37 patients. From April 2003 to September 2007, PVE was performed using gelfoam (n = 17) and a gelfoam-coil combination (n = 20) to induce hypertrophy. Computed tomography volumetry was performed 2-4 weeks after PVE to assess the changes in liver volume.
Results
The mean percentage increase in future liver remnant volume was 23.7 ± 23.7% in the gelfoam group and 36.7 ± 18.5% in the gelfoam-coil group (p = 0.02). Recanalization was found in 15 gelfoam group patients and 8 gelfoam-coil group patients (p = 0.003). The mean tumor size increased from 4.5 ± 2.9 cm before PVE to 5.0 ± 3.5 cm after PVE in the gelfoam group and from 4.3 ± 2.2 cm before PVE to 4.7 ± 2.5 cm after PVE in the gelfoam-coil group (p = 0.80).
Hepatocellular carcinoma (HCC) is the fifth most common neoplasm in the world, and the third most common cause of cancer-related death (1). According to the Barcelona Clinic Liver Cancer staging classification and treatment schedule, surgical resection is considered the first treatment option for patients with early-stage HCC (2). However, liver resection may be contraindicated if the future liver remnant (FLR) volume is not sufficient to avoid post-hepatectomy liver failure. Patients treated with portal vein embolization (PVE) before a major liver resection for HCC show fewer postoperative complications and better cumulative survival rates than those who have not received PVE (3, 4). The minimal FLR volume required following liver resection is > 25% in patients with normal livers and > 40% in those with chronic liver disease (5), and liver regeneration is slower in the non-embolized lobe paired with an injured liver than in the normal liver (6, 7). Therefore, more efficient PVE is necessary for patients with chronic liver disease.
To date, various embolic materials including absorbable gelatin sponges (gelfoam), coils, thrombin, polyvinyl alcohol particles, absolute alcohol, and N-butyl cyanoacrylate have been used for PVE (8). A few animal studies showed that PVE using permanent embolic substances is more effective than PVE using temporary substances (9, 10). Although PVE has been performed using gelfoam and coils (11,12,13), few reports exist regarding the comparison of the effectiveness of gelfoam alone and a gelfoam-coil combination in PVE. Therefore, the purpose of this study was to compare the effectiveness of PVE performed using gelfoam alone and a gelfoam-coil combination in patients with HCC.
This study was performed with the approval of our Institutional Review Board, which waived patient informed consent owing to the retrospective study design. From April 2003 to September 2007, a total of 55 patients with HCC underwent PVE for induction of selective hepatic hypertrophy before major hepatic resection in our institution. Eighteen patients were excluded because of the absence of measurement data. Therefore, 37 patients (32 men and 5 women, mean age: 53.4 years, range 34-79 years) with 43 HCCs were enrolled in this study. Patients were divided into two groups. From April 2003 to April 2005, 17 patients with HCC underwent PVE with gelfoam alone [gelfoam group (GG)] and from May 2005 and September 2007, 20 patients with HCC underwent PVE with a gelfoam-coil combination [gelfoam-coil group (GCG)]. The clinical characteristics and tumor burden (size and number) of the patients are summarized in Table 1. HCC was confirmed histopathologically in 37 tumors from 32 patients who underwent hepatic resection, and six tumors of five patients who did not undergo hepatic resection were considered to be HCC based on the imaging and laboratory findings according to the American Association for the Study of Liver Disease guidelines. The diagnosis of HCC was based on biopsy or imaging findings that showed intense arterial uptake followed by washout of contrast in the venous-delayed phase on CT or MRI (14).
It has been reported that CT volumetry can accurately assess liver volumes (5). In all patients, CT scanning was performed before and after PVE. All CT examinations before and after PVE were performed using one of four helical scanners (LightSpeed QX/1, LightSpeed16, or LightSpeed Ultra; GE Medical Systems, Milwaukee, WI, USA or Aquilion; Toshiba Medical Systems Corporation, Tokyo, Japan). A total of 120 mL of nonionic contrast materials (Iopromide; Ultravist 300, Shering, Berlin, Germany) was injected with an automatic injector (OP 100; Medrad, Indianola, PA, USA) at a rate of 3 mL/s. The images were obtained at 25-35, 60-70, and 180 s after the initiation of contrast material injection, representing the hepatic arterial, portal venous, and equilibrium phase, respectively. The parameters for the single detector helical CT scanner and multidetector CT were 7.0-mm slice thickness and 7.0-mm interval and 2.5-5.0-mm slice thickness and 2.5-5.0-mm intervals, respectively.
Volumetry was performed using a three-dimensional CT analysis program (Virtual Place Advance Plus version 2.03; Aze Co., Tokyo, Japan). Each liver slice was traced with a cursor and the corresponding area was calculated by computer exclusion of the inferior vena cava, main portal vein, and gallbladder. The middle hepatic vein and gallbladder were used as landmarks to define the borders between the right and left liver. The caudate lobe was calculated as a part of the left liver because its portal vein was not embolized. The volume of liver segment IV was measured with the middle hepatic vein and the umbilical portion of the left portal vein as landmarks. The total liver volume (TLV), right liver volume (RLV), percentage FLR (% FLR), and percentage increase of FLR volume (% increase of FLR volume) were obtained from CT volumetry measurements. The FLR volume was considered the volume of the left liver (segments I-IV) in 25 patients who underwent right lobectomy and the left lateral segment (segments I-III) in 7 patients who underwent extended right lobectomy. The % FLR was calculated according to the following formula:
The % increase of FLR volume after PVE was calculated according to the following formula:
PVE was performed if the FLR volume was < 30% of the calculated TLV. The indocyanine green (ICG) retention test (ICG-R15; ICG retention rate 15 min after injection of a 0.5-mg/kg dose < 15%) was a prerequisite for major hepatic resection in our institution. PVE was performed by one of six fellowship-trained interventional radiologists in our hospital.
PVE was performed under intravenous conscious sedation with 50 mg of pethidine hydrochloride (Pethidine; Samsung Pharmaceutical, Seoul, Korea). For pain control, 1% lidocaine (Kwang Myung Pharmaceutical, Seoul, Korea) was injected through the skin into the liver capsule along a predetermined puncture route. Routine preprocedural antibiotics were not administered to the patients. During the procedure, vital signs were monitored continuously. Access to the portal vein was obtained by percutaneous placement of a 22-gauge needle (Chiba needle, 22-G, 15 cm; Cook, Bloomington, IN, USA) into either the right or left portal vein under ultrasonographic and fluoroscopic guidance. The right or left percutaneous transhepatic approach was performed in 25 and 12 patients, respectively, on the basis of operator preference. The Seldinger technique was used to place a 5-Fr vascular sheath (Radiofocus; Terumo, Tokyo, Japan) into the right or left portal vein. Portography was performed to identify individual branches and anatomic variations of the portal vein using a 5-Fr catheter (Cobra 2; Cook, Bloomington, IN, USA). A gelatin sponge (Gelfoam; Upjohn, Kalamazoo, MI, USA), which was cut into 1-2-mm sections and then slurried by vigorous manual pumping between two 10-mL syringes, was used to embolize the portal vein branches (Fig. 1). In the GCG, at least two 0.035-inch metallic coils (Nester; Cook, Bloomington, IN, USA) that were 3-8 mm in diameter and 14 cm in length were placed within the first- or second-order branches of the right portal vein to prevent recanalization of the portal vein (Fig. 2). Embolization was performed until stasis of portal vein flow was achieved. Completion portography was obtained with the catheter positioned in the main portal vein to assess completeness of the embolization. The access tract was embolized with a gelatin sponge or coils on completion of the procedure.
Technical success was defined as completion of embolization of the portal vein in the hepatic lobe to be resected. Complications after PVE and liver resection were evaluated. The major and minor complications were defined according to the standard terminology and reporting criteria of the Society of Interventional and the Radiology Technology Assessment Committee (15).
Routine laboratory tests for liver function, including measurement of aspartate aminotransferase, alanine aminotransferase, and total bilirubin, were performed before and after PVE. The follow-up liver function test was performed 1-30 days (mean: 7.5 ± 10.5 days, range: 1-30 days in GG, mean: 7.8 ± 9.5 days, range 1-28 days in GCG) after PVE.
All patients underwent follow-up contrast-enhanced three-phase CT. The post-PVE follow-up was performed 2-4 weeks (mean: 20.9 ± 5.4 days, range: 13-33 days in GG, mean 18 ± 4.4 days, range: 8-30 days in GCG, p = 0.117) after PVE and hepatic resection was performed within 2-6 weeks (mean: 24.2 days, range: 14-48 days) after PVE. If there were no complications, the patient was discharged 2 days after PVE.
All data are expressed as mean ± standard deviation. The Mann-Whitney U test was used to analyze the differences between two groups in TLV, RLV, % FLR, and the % increase of FLR volume after PVE, as well as the tumor size before and after PVE. Comparison of the recanalization rate between two groups was performed using the chi-square test. A p value < 0.05 was considered statistically significant. Statistical analysis was performed using commercially available statistics software (Statistical Package for the Social Sciences for Windows, version 17.0, SPSS Inc.; Chicago, IL, USA).
Primary technical success of PVE was achieved in all patients. There were no complications related to the procedure. Five patients (13.5%, 5/37) did not undergo hepatic resection after PVE for the following reasons: extrahepatic metastasis (n = 2), intrahepatic metastasis (n = 2), and aggravation of hyperbilirubinemia caused by bile duct invasion (n = 1). Therefore, 32 patients subsequently underwent hepatectomy (right hepatectomy: 25, extended right hepatectomy: 7) after PVE.
The median hospital stay after PVE was 1.6 ± 0.9 days (range: 1-5 days) and there was no significant difference between the two groups (p = 0.94). Pre- and post-PVE laboratory tests for liver function were similar in both groups (Table 2).
There were no operative mortalities or major complications in either the GG or GCG after PVE and surgery. There were several minor postoperative complications, including wound problems such as seroma (n = 4), pleural effusion (n = 1), and peritoneal fluid collection with fever (n = 1). These patients with minor complications were discharged within 3 weeks.
There was no significant difference in TLV, FLR before PVE, and % FLR between the GG and GCG (Table 3).
The FLR volume increased from 365.4 ± 114.8 cm3 (range 118-559.7 cm3) to 440.8 ± 114.8 cm3 (range 191.1-743.7 cm3) after PVE in GG and from 358.4 ± 121.6 cm3 (range 174.3-673.5 cm3) to 480.2 ± 140.3 cm3 (range 220.9-730.4 cm3) after PVE in GCG. The increased FLR volume was significantly different between the two groups (p = 0.02). The mean % increase of FLR volume induced by PVE was 23.7 ± 23.7% 1-70.9%) in GG and 36.7 ± 18.5% (range 8.4-83.2%) in GCG, respectively (p = 0.02) (Table 3).
Portal vein recanalization after PVE was observed in 15/17 (88.2%) patients in GG and in 8/20 (40%) patients in GCG, respectively (p = 0.003). Complete recanalization of the portal vein was observed in 14/15 (93.3%) patients in GG and 3/8 (37.5%) patients in GCG. The mean % increase of FLR volume was 29.9 ± 26.12% in the recanalization group and 37 ± 15.6% in the non-recanalization group (p = 0.219).
The mean tumor size increased from 4.5 ± 2.9 cm before PVE to 5.0 ± 3.5 cm after PVE in the GG and from 4.3 ± 2.2 cm before PVE to 4.7 ± 2.5 cm after PVE in the GCG. There was no significant difference in tumor size changes between the groups before and after PVE (Table 4). The mean tumor size increased from 4.2 ± 2.5 cm before PVE to 4.6 ± 2.9 cm in the recanalization group and from 4.9 ± 2.7 cm before PVE to 5.3 ± 3.2 cm in the non-recanalization group (p = 0.816).
Since its first clinical application in patients with hilar bile duct cancer to induce compensatory FLR hypertrophy (16), PVE has become a safe and effective procedure for preventing post-resection liver failure due to an insufficient liver remnant (8). Although the normal liver is known to tolerate removal of up to 60% of its volume (5), major hepatectomy in patients with chronic liver disease such as chronic hepatitis or liver cirrhosis is related to the risk of hepatic failure due to impaired liver regeneration (17). In addition, the rate of postoperative complications is significantly reduced after preoperative PVE in patients with chronic liver disease (18). These reports suggest that PVE is particularly advantageous in patients with liver disease.
A few studies have compared the effectiveness of different embolic materials using animal models to determine the material that provides the highest rate of hypertrophy of the non-embolized portion of the liver. Huang et al. (10) reported that PVE using permanent embolic materials such as absolute ethanol or a gelfoam-coil combination can induce compensatory hypertrophy, but gelfoam alone is an inefficient embolic material. Our study showed that total recanalization and the complete recanalization rate of the portal vein after PVE were 88.2% and 93.3% in GG patients, respectively. These results support the findings of previous studies that have shown the limitation of gelfoam in achieving complete PVE. De Baere et al. (9) reported that N-butyl cyanoacrylate induces a significantly greater increase in the size of hepatic lobules of hypertrophy than other embolic materials such as hydrophilic gel and polyvinyl alcohol. Gelfoam-coil combinations have been used clinically as embolic materials for PVE in several previous studies (11, 12, 13, 19, 20), but few studies have compared the efficacy between permanent and temporary embolic materials. Some interventional radiologists in our institution performed PVE with gelfoam alone because of a few advantages, including safety, low price, easy handling, and minimal inflammatory reaction. In addition, they thought that even if migration of gelfoam into portal branches of the contralateral lobe occurred, there would be minimal or no effect on the contralateral lobe because gelfoam is an absorbable biomaterial. However, initial studies with gelfoam reported frequent recanalization (16, 21) and less hypertrophy compared with permanent embolic materials (22). Therefore, the other interventional radiologists in our hospital added coils as embolic materials to prevent recanalization by occlusion of proximal portal blood flow. In addition, an experimental study reported that proximal and complete revascularization occurred 6-8 and 12-16 days after PVE using gelfoam (23). De Baere et al. (22) demonstrated that a key factor in achieving satisfactory hypertrophy of the non-embolized liver is complete and durable occlusion of the portal vein, and showed that cyanoacrylate seemed to induce better and faster hypertrophy than gelfoam or coils alone. In the same study, coils alone showed worse results in compensatory hypertrophy than gelfoam. The result could be explained by distal re-entry through the intraparenchymatous vascular shunt, which opened after proximal portal vein occlusion. To prevent this, the distal portal venous branch should be occluded with other embolic materials such as gelfoam or polyvinyl alcohol.
Our study showed that PVE using a gelfoam-coil combination in patients with chronic liver disease is more likely to induce greater compensatory hypertrophy of the non-embolized portion of the liver than PVE using gelfoam alone. Furthermore, PVE using a gelfoam-coil combination showed no significant differences in postprocedural complications. Therefore, we believe that PVE should be performed using permanent embolic materials in patients with liver damage.
Since the first description of the potential of intrahepatic tumor enlargement after PVE (24), accumulating evidence has shown that PVE stimulates tumor growth in both embolized and non-embolized liver segments (13, 25). Hayashi et al. (26) reported that the median tumor growth rate of primary liver cancer in the embolized lobe after PVE was approximately two times greater than that before PVE. Three possible mechanisms have been proposed: changes in cytokine and growth factor secretion, alteration in hepatic blood flow, and enhanced cellular host response that promotes local tumor growth (27). To the best of our knowledge, few studies have compared the tumor growth rate according to the embolic materials used. There was no significant difference in tumor growth rate between the two groups in this study.
The limitations of this study include the following. First, it is a retrospective study. Second, there may be a gap between the estimated FLR volume using CT volumetry according to Couinaud's classification and the actual FLR volume. Fischer et al. (28) showed that the segmental volume was overestimated by the classic Couinaud's method by up to 24% and underestimated by 13%.
In conclusion, this study demonstrates that PVE using a gelfoam-coil combination before major hepatectomy for HCC is more effective than PVE using gelfoam alone for induction of compensatory hypertrophy and there is no significant tumor growth rate after PVE between two groups.
Figures and Tables
Fig. 1
A 45-year-old man with a hepatocellular carcinoma underwent preoperative portal vein embolization using only gelfoam.
A. Frontal portography before embolization shows intact right portal vein.
B. Frontal portography after embolization using gelfoam only shows occlusion of right portal vein and redirection of portal blood flow to left portal vein.
C. Abdominal CT scan obtained 4 weeks after portal vein embolization shows recanalization of right portal vein.

Fig. 2
A 46-year-old man with a hepatocellular carcinoma underwent preoperative portal vein embolization using gelfoam-coil combination.
A. Frontal portography before embolization shows patent right portal vein.
B. Frontal portography after embolization using gelfoam-coil combination shows complete occlusion of right portal vein.
C, D. Abdominal CT scan obtained before PVE (C) and 3 weeks after PVE (D) at the level of celiac trunk shows hypertophic change of left lobe and hepatic attenuation difference and hypotrphic change of right lobe (arrows) suggesting embolized state of right lobe of the liver. The FLRbefore PVE was 431.7 cm3 and the FLRafter PVE was 714 cm3. The % increase of FLR volume after PVE was 65.4%.
FLR = future liver remnant, PVE = portal vein embolization
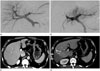
Table 1
Summary of the Patient Characteristics

Table 2
Comparison of Liver Function Test between Gelfoam and Gelfoam-Coil Groups
Table 3
Comparison of Liver Volume Changes before and after PVE between Gelfoam and Gelfoam-Coil Groups

References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001; 94:153–156.
2. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010; 30:61–74.
3. Tanaka H, Hirohashi K, Kubo S, Shuto T, Higaki I, Kinoshita H. Preoperative portal vein embolization improves prognosis after right hepatectomy for hepatocellular carcinoma in patients with impaired hepatic function. Br J Surg. 2000; 87:879–882.
4. Denys A, Lacombe C, Schneider F, Madoff DC, Doenz F, Qanadli SD, et al. Portal vein embolization with N-butyl cyanoacrylate before partial hepatectomy in patients with hepatocellular carcinoma and underlying cirrhosis or advanced fibrosis. J Vasc Interv Radiol. 2005; 16:1667–1674.
5. Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997; 26:1176–1181.
6. Lee KC, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993; 17:109–115.
7. Madoff DC, Hicks ME, Vauthey JN, Charnsangavej C, Morello FA Jr, Ahrar K, et al. Transhepatic portal vein embolization: anatomy, indications, and technical considerations. Radiographics. 2002; 22:1063–1076.
8. Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008; 247:49–57.
9. de Baere T, Denys A, Paradis V. Comparison of four embolic materials for portal vein embolization: experimental study in pigs. Eur Radiol. 2009; 19:1435–1442.
10. Huang JY, Yang WZ, Li JJ, Jiang N, Zheng QB. Portal vein embolization induces compensatory hypertrophy of remnant liver. World J Gastroenterol. 2006; 12:408–414.
11. Wakabayashi H, Okada S, Maeba T, Maeta H. Effect of preoperative portal vein embolization on major hepatectomy for advanced-stage hepatocellular carcinomas in injured livers: a preliminary report. Surg Today. 1997; 27:403–410.
12. Wakabayashi H, Ishimura K, Okano K, Karasawa Y, Goda F, Maeba T, et al. Application of preoperative portal vein embolization before major hepatic resection in patients with normal or abnormal liver parenchyma. Surgery. 2002; 131:26–33.
13. Kokudo N, Tada K, Seki M, Ohta H, Azekura K, Ueno M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology. 2001; 34:267–272.
14. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.
15. Omary RA, Bettmann MA, Cardella JF, Bakal CW, Schwartzberg MS, Sacks D, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2002; 13(9 Pt 1):879–881.
16. Makuuchi M, Takayasu K, Takuma T, Yamazaki S, Hasegawa H, Nishiura S, et al. Preoperative transcatheter embolization of the portal venous branch for patients receiving extended lobectomy due to the bile duct carcinoma. J Jpn Soc Clin Surg. 1984; 45:1558–1564.
17. Lin TY, Chen CC. Metabolic function and regeneration of cirrhotic and non-cirrhotic livers after hepatic lobectomy in man. Ann Surg. 1965; 162:959–972.
18. Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003; 237:208–217.
19. Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000; 127:512–519.
20. Kim MJ, Choo SW, Do YS, Park KB, Han YH, Choo IW, et al. Use of double-occlusion balloon catheter: preoperative portal vein embolization for induction of future remnant liver hypertrophy. Cardiovasc Intervent Radiol. 2004; 27:16–20.
21. de Baere T, Roche A, Vavasseur D, Therasse E, Indushekar S, Elias D, et al. Portal vein embolization: utility for inducing left hepatic lobe hypertrophy before surgery. Radiology. 1993; 188:73–77.
22. de Baere T, Roche A, Elias D, Lasser P, Lagrange C, Bousson V. Preoperative portal vein embolization for extension of hepatectomy indications. Hepatology. 1996; 24:1386–1391.
23. Lainas P, Boudechiche L, Osorio A, Coulomb A, Weber A, Pariente D, et al. Liver regeneration and recanalization time course following reversible portal vein embolization. J Hepatol. 2008; 49:354–362.
24. Elias D, De Baere T, Roche A, Mducreux , Leclere J, Lasser P. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg. 1999; 86:784–788.
25. van Gulik TM, van den Esschert JW, de Graaf W, van Lienden KP, Busch OR, Heger M, et al. Controversies in the use of portal vein embolization. Dig Surg. 2008; 25:436–444.
26. Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007; 48:721–727.
27. de Graaf W, van den Esschert JW, van Lienden KP, van Gulik TM. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol. 2009; 16:423–430.
28. Fischer L, Cardenas C, Thorn M, Benner A, Grenacher L, Vetter M, et al. Limits of Couinaud's liver segment classification: a quantitative computer-based three-dimensional analysis. J Comput Assist Tomogr. 2002; 26:962–967.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download