Abstract
Chondrosarcoma is a commonly encountered malignant cartilaginous tumor. However, only 1% of chondrosarcomas arise in the extraskeletal region. The pathologic types of this tumor include mesenchymal, myxoid, and low grade. A mesenchymal chondrosarcoma is a rare, highly malignant cartilaginous tumor that is rarely encountered, and it shows similar imaging features to other malignant soft-tissue tumors. Here, we report a mesenchymal chondrosarcoma presenting as a palpable mass in the neck, arising in the carotid space, which is also known as the retrostyloid parapharyngeal space.
Chondrosarcoma is the third most common malignant bone tumor, with extraskeletal chondrosarcoma accounting for only 1% of all reported chondrosarcoma cases (1). Since it was first described in 1959 by Lightenstein and Bernstein (2), mesenchymal chondrosarcoma (MC) is considered as a subtype of chondrosarcoma that represents less than 10% of all chondrosarcomas (3). MC is assumed to arise from remnants of the embryonic cartilage or metaplasia of meningeal fibroblasts (4). The incidence of extraskeletal cases of MC is only one half that of skeletal cases (3). Typically involving the extraskeletal regions, MCs have been documented in the central nervous system, meninges, maxillary sinuses, eyelid, eye socket, and thyroid (1). No report has described chondrosarcoma in the carotid space, and it may be difficult to identify chondrosarcoma based on differential diagnosis of a soft-tissue mass. Therefore, we report here a very rare case of extraskeletal MC (EMC) originating from the carotid space, and its radiological features and possible differential diagnosis will be discussed. This report was approved by the Institutional Review Board.
A 64-year-old man presented with a gradually growing, palpable neck mass and 11 kg of weight loss in 9 months. A physical examination revealed a non-tender, palpable, growing mass in the left submandibular area. He had no remarkable medical history.
Unenhanced computed tomography (CT) showed a lobulating, contoured, well-circumscribed isodense mass approximately 4.8 × 3.2 cm in size with dense ring-and-arc-patterned calcifications in the posterior aspect of the left carotid space at the carotid bifurcation level. Precontrast and postcontrast CT revealed heterogeneous enhancement, and the mass encased the left common, internal, and external carotid arteries with splaying internal and external carotid arteries containing calcifications within them (Fig. 1). Based on dense calcifications with a ring-and-arc pattern representing chondroid calcifications, a cartilage-derived tumor, such as chondrosarcoma, was suspected. In addition, the location of the carotid space, known as the retrostyloid parapharyngeal space, suggested other tumors such as paraganglioma and schwannoma (5).
Under the suspicion of these tumors, contrast-enhanced magnetic resonance imaging (MRI) was performed for more detailed evaluation, particularly to differentiate among chondrosarcoma, glomus jugulare, and schwannoma. MRI demonstrated a large, aggressive mass centered in the left carotid space, similar to that observed on CT. On T2-weighted imaging, the lesion showed heterogeneous hyperintensity with heterogeneously low-signal foci representing a chondroid matrix, invasion of surrounding structures with extension to the lowest crotch of carotid bifurcation, and encasement of the left external and internal carotid arteries (Fig. 2A). The lesion showed isosignal intensity on T1-weighted imaging with low-signal foci and revealed marked enhancement following intravenous gadolinium administration (Fig. 2B). On diffusion-weighted imaging (Fig. 2C) and apparent diffusion coefficient mapping, this mass showed heterogeneously high-signal intensity and diffusion restriction, respectively, representing high cellularity of tumor suggestive of malignant character. Surgical resection planning of this lesion was very complicated, because the location required carotid resection and vessel replacement. Ultrasonography-guided gun biopsy was carried out before detailed planning of the treatment options.
Microscopically, sections showed a characteristic bimorphic structure consisting of hypercellular, undifferentiated tumor cells and a well-defined nodule of well-differentiated cartilage. Undifferentiated tumor cells have ovoid or elongated hyperchromatic nuclei and scanty, poorly outlined cytoplasm (hematoxylin-eosin, × 200) (Fig. 3A), and the chondroid component show well differentiated cartilage (hematoxylin-eosin, × 100) (Fig. 3B). The high cellularity was compatible with imaging findings, which showed diffusion restriction. The undifferentiated tumor cells showed ovoid or elongated hyperchromatic nuclei and poorly outlined cytoplasm with diffuse positivity on immunohistochemical staining of CD99; the chondroid component showed well-differentiated cartilage with diffuse positivity on immunohistochemical staining of S-100 protein.
Most of the masses in the carotid space are benign tumors such as paragangliomas or schwannomas (5). Paraganglioma, also known as chemodectoma or a glomus tumor, is a hypervascular tumor that most commonly arises in the carotid artery bifurcation, as in our case. Typically, a glomus tumor presents as an intensely enhanced, well-marginated mass. The most characteristic MR finding of this tumor is a serpentine or punctuate and low-signal-intensity lesion or signal voids. If the greater diameter is larger than 1.5-2.0 cm, the tumor should have these lesions. With these signal-voided lesions and adjacent high-signal areas, the adjacent high- and low-intensity regions have been termed as having a "salt-and-pepper" appearance (5). Even with a maximum diameter of 4.8 cm, our case did not demonstrate this characteristic feature, so the possibility of a paraganglioma such as a glomus jugulare would be very low.
The second most common benign tumor of the carotid space is a nerve sheath tumor, consisting of a schwannoma or a neurofibroma. A schwannoma is defined as a tumor arising from Schwann cells of the nerve sheath, and it has both the solid fibrous and gelatinous components. In this region, the most common site is the vagus nerve followed by the sympathetic trunk and other adjacent nerves. If the tumor originates from the vagus nerve, the tumor displaces the internal carotid artery anteromedially and internal jugular vein posterolaterally. If the tumor originates from the lower cranial nerves, neurogenic tumors do not separate them but only displace the carotid vessels posterolaterally (5). A schwannoma usually shows higher attenuation on contrast-enhanced CT, but it may be isodense or even show lower attenuation than adjacent muscle. On MRI, a schwannoma is a homogeneous, significantly enhanced mass with areas of necrosis or hemorrhage. However, schwannomas do not have a salt-and-pepper appearance as noted with paragangliomas (6).
Most common malignant tumors in the parapharyngeal space are direct extensions from nasopharyngeal or tonsillar carcinoma (5). Without any underlying primary cancer in our case, we thought the possibility of metastasis would be very low. The common benign or malignant tumors that are known did not correspond with our case; thus, we thought the differential diagnosis should be based on the characteristic imaging findings alone and not on the typical location. The most striking finding was the dense calcifications in a ring-and-arc pattern, which are the characteristic findings of a cartilage-derived tumor such as a chondrosarcoma (7). An MC is a highly malignant, cartilage-derived tumor that forms in bone or soft tissue. In addition, with typical chondroid matrix mineralization such as calcifications showing a ring-and-arc pattern, radiographic findings strongly suggest the diagnosis of chondrosarcoma, which includes a heterogeneous group of lesions with various clinical behaviors and morphological features (7). Additional diagnostic clues can be obtained with MR images. Calcified areas show low intensity in both T1-weighted images (T1WI) and T2-weighted images (T2WI), whereas uncalcified areas show low T1 and high T2 signal intensities. However, these features depend greatly on the extent or pattern of intra-tumoral calcifications. When tumors have a clear demarcation between the calcified focus and unmineralized tumor tissue, MRI shows more characteristic features, particularly on T2WI. This is because the contrast is more evident, than in T1WI, due to the higher intensity of the surrounding unmineralized tissues. Thus, low-intensity areas are located in the surrounding unmineralized T2 high-intensity area, forming a very impressive image (8).
Unless imaging findings of MC are evident, such as an aggressive lesion with a ring-and-arc pattern or stipple pattern of calcifications, it is impossible to differentiate this neoplasm from a conventional chondrosarcoma. Thus, a correlation with anatomic distribution and clinical findings should be considered to achieve the most likely diagnosis. EMC differs from conventional chondrosarcoma. For example, there is a slight female predominance in EMC, which originates frequently in the brain, meninges, and soft tissue of the face and lower extremities; whereas, conventional chondrosarcomas are usually found in the pelvis and femur (9).
Originally identified by Lightenstein and Bernstein (2) in 1959, only about 300 cases of EMC have been reported in the literature, and no reported case of this tumor has arisen from the carotid space (10). Despite this low prevalence, noticing this neoplasm is very important because of its presence in younger populations, aggressive nature, and requirement of radical excision therapy (10). Because MC has a high histological grade, its prognosis is generally poor with local recurrence and metastasis being common. Thus, unfortunately, the median survival is just over 3 years with 5- and 10-year survival rates of 42% and 28%, respectively (9, 10).
Hence, EMC should be diagnosed accurately and as early as possible. The combination of typical imaging features, such as dense, ring-and-arc calcifications, and high-signal intensity surrounding low-signal intensity on T2WI, can enhance the accuracy of diagnosis even if the anatomical location is not typical.
In conclusion, EMC is a rare tumor that can involve the head and neck area; in our case, the carotid space was involved. The tumor is a lobulating, contoured, well-circumscribed mass, with iso-signal intensity on T1WI and heterogeneously high-signal intensity with heterogeneous low-signal intensity foci representing a chondroid matrix on T2WI. The mass showed significant contrast enhancement on both CT and MRI. Soft-tissue tumors such as schwannomas or paragangliomas can be considered as a possible differential diagnosis in a mass arising in the carotid space. However, when the mass shows a characteristic ring-and-arc pattern representing chondroid calcifications in CT and MRI findings, chondrosarcomas should also be considered.
Figures and Tables
Fig. 1
A 64-year-old man presented with a gradually increasing, palpable neck mass and 11 kg weight loss in 9 months.
A. Precontrast CT reveals soft tissue density mass in the left carotid space. Dense calcifications with a ring-and-arc pattern representing chondroid calcifications is noted within the mass.
B. Contrast enhanced CT reveals heterogeneous enhancing solid mass encasing the left common, internal, and external carotid arteries with splaying internal and external carotid arteries.
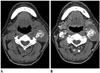
Fig. 2
The MR images of 64-year-old man with extraskeletal mesenchymal chondrosarcoma of the carotid space.
A. The lesion shows heterogeneously hyperintense with heterogeneously low-signal foci representing a chondroid matrix on T2-weighted imaging and reveals invasion of surrounding structures with extension to the lowest crotch of the carotid bifurcation and encasement of the left external and internal carotid arteries.
B. Contrast enhanced axial MRI scan shows well enhancing heterogeneous mass with low signal intensity foci in the left carotid space with splaying internal carotid artery and external carotid artery. The lesion shows isosignal intensity on T1-weighted imaging with low-signal-intensity foci (not shown).
C. On diffusion-weighted imaging, this mass shows heterogeneous high-signal intensity and diffusion restriction on apparent diffusion coefficient mapping (not shown), suggestive of a malignant character of the tumor.
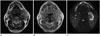
Fig. 3
Histopathological findings have a characteristic bimophic structure.
A. One core reveals sheets of hypercellular, undifferentiated tumor cells. The other core shows a well-defined nodule of well-differentiated cartilage. A undifferentiated tumor cells have ovoid or elongated hyperchromatic nuclei and scanty, poorly outlined cytoplasm (hematoxylin-eosin, × 200).
B. Chondroid component show well differentiated cartilage (hematoxylin-eosin, × 100).

References
1. Shapeero LG, Vanel D, Couanet D, Contesso G, Ackerman LV. Extraskeletal mesenchymal chondrosarcoma. Radiology. 1993; 186:819–826.
2. Lightenstein L, Bernstein D. Unusual benign and malignant chondroid tumors of bone. A survey of some mesenchymal cartilage tumors and malignant chondroblastic tumors, including a few multicentric ones, as well as many atypical benign chondroblastomas and chondromyxoid fibromas. Cancer. 1959; 12:1142–1157.
3. Nakashima Y, Unni KK, Shives TC, Swee RG, Dahlin DC. Mesenchymal chondrosarcoma of bone and soft tissue. A review of 111 cases. Cancer. 1986; 57:2444–2453.
4. Bahr AL, Gayler BW. Cranial chondrosarcomas. Report of four cases and review of the literature. Radiology. 1977; 124:151–156.
5. Mafee MF, Valbasson GE, Becker M. Imaging of the Head and Neck. Stuttgart: Georg Thieme Verlag;2012. p. 602–608.
6. Som PM, Curtin HD. Head and Neck Imaging. Boston: Elsevier Health Sciences;2011. p. 1749–1779.
7. Murphey MD, Walker EA, Wilson AJ, Kransdorf MJ, Temple HT, Gannon FH. From the archives of the AFIP: imaging of primary chondrosarcoma: radiologic-pathologic correlation. Radiographics. 2003; 23:1245–1278.
8. Hashimoto N, Ueda T, Joyama S, Araki N, Beppu Y, Tatezaki S, et al. Extraskeletal mesenchymal chondrosarcoma: an imaging review of ten new patients. Skeletal Radiol. 2005; 34:785–792.
9. Huvos AG, Rosen G, Dabska M, Marcove RC. Mesenchymal chondrosarcoma. A clinicopathologic analysis of 35 patients with emphasis on treatment. Cancer. 1983; 51:1230–1237.
10. Banks KP, Ly JQ, Thompson LD, Michaelson PG, Davis SW. Mesenchymal chondrosarcoma of sinonasal cavity: a case report and brief literature review. Eur J Radiol Extra. 2004; 49:47–51.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download