Abstract
Parathyromatosis is characterized by multiple lesions of benign hyperfunctioning parathyroid tissue in the neck or mediastinum. Parathyromatosis is caused by proliferation of embryonic remnant or seeding of parathyroid tissue after parathyroidectomy. Parathyromatosis is rare but is the common cause of recurrent hyperparathyroidism. We describe a unique case of non-functional parathyromatosis in a 16-year-old girl with a history of right thyroid lobectomy via an axillo-breast approach for a nonfunctioning parathyroid adenoma in the right side of the neck.
Parathyromatosis is a rare disease that occurs in the neck and mediastinum as multiple lesions leading to hyperparathyroidism (1, 2). Parathyromatosis is usually divided into 2 types: primary parathyromatosis, which is proliferation of an embryonic remnant, and the more common secondary parathyromatosis, which is a post-surgical growth of retained parathyroid tissues or seeding of parathyroid tissue by capsular rupture of parathyroid adenoma during parathyroidectomy (1, 2). Early diagnosis and treatment of parathyromatosis is important because parathyromatosis also causes recurrent metabolic disorder after a parathyroidectomy for hyperparathyroidism (1, 2, 3). We experienced a patient with parathyromatosis without hyperparathyroidism. In this study, we report a case of secondary parathyromatosis after endoscopic thyroidectomy for a presumed thyroid mass via an axillo-breast approach.
A 16-year-old girl visited our institution for treatment of palpable masses in the anterior neck and upper chest wall. She had previously received an endoscopic right thyroid lobectomy via an axillo-breast approach for a painless palpable mass on the right side of the neck at an outside institution 6 months ago. A contrast-enhanced dynamic neck computed tomography (CT) with 2 phase (arterial phase 30 second, delayed phase 150 second) scan performed during the initial visit showed enhanced right thyroid tumor with early enhancement and delayed wash-out (Fig. 1). Ultrasonography-guided fine needle aspiration (FNA) was performed immediately and the pathologic report of FNA was follicular proliferation. The surgery was performed on the presumptive diagnosis of a tumor originating from the thyroid. Pathologic examination from the surgical exploration revealed a parathyroid adenoma. One month post-operation, palpable lesions appeared on her neck and appeared to increase in size and number. All chemistry and hematology tests, including serum phosphate (3.4 mg/dL) and calcium (9.3 mg/dL), and parathyroid hormone (42.63 pg/mL), were within normal range during her preoperative work up at our institution. Her mother had an operation for a benign lesion in the thyroid gland. The girl's past history was unremarkable.
Ultrasonography showed multiple, ovoid, homogeneous, hypoechoic masses. Color Doppler ultrasonography revealed increased vascularity within masses in the neck and left anterior chest wall (Fig. 2). Contrast-enhanced dynamic neck CT showed multiple homogeneous early enhancing nodular lesions with delayed wash-out measuring 2-15 mm in diameter in the subcutaneous fatty layer of the anterior neck, left upper chest wall, right thyroid lobectomy site, and anterior and posterior to the strap musculature (Fig. 3). A technetium 99m tetrofosmin dual phase washout parathyroid gland scan showed delayed multiple tracer activity in the anterior neck and left upper chest wall (Fig. 4).
En-bloc excision of multiple masses and a left thyroid lobectomy was done with central node dissection. Pathologic specimens of the multiple masses showed clear cells with clear cytoplasm without atypia or invasion that stained positive for parathyroid hormone. Twenty months later, no tumor recurrence was detected during the follow-up examinations.
Parathyromatosis is a rare disease with scattered hyperfunctioning parathyroid tissues throughout the neck and mediastinum (1, 4). There are 3 theories regarding the origin of parathyromatosis. First is parathyroid malignancy, i.e., scattered or diffuse implantation of malignant cells (4). Second is seeding after spillage of parathyroid tissue from the encapsulated parathyroid gland during parathyroid surgery (5). Third is an overgrowth of embryologic remnant of parathyroid tissue (6). This occurs most frequently after previous parathyroid surgery in patients with hyperparathyroidism and also occurs after FNA (2). Palmer et al. (5) described parathyromatosis first in 1975 and Reddick et al. (6) classified it as a disease entity. Parathyromatosis is usually found at the site of operation in the fibrofatty tissue and muscle straps, the thyroid gland, and the superior mediastinum (5, 6). Parathyromatosis is an important cause of recurrent or persistent hyperparathyroidism after parathyroid surgery, especially in patients with secondary hyperparathyroidism resulting from chronic renal failure that stimulates proliferation of remnant parathyroid tissue (3). History of trauma and surgery, together with laboratory examinations, are helpful for differential diagnosis (4). Blood calcium levels in patients with parathyromatosis are significantly lower than in patients with parathyroid carcinoma (4).
Preoperative diagnosis of parathyromatosis is difficult. Most diagnoses of parathyromatosis are by intraoperative findings. Parathyromatosis is usually suspected when hyperparathyroidism accompanies elevated calcium and parathyroid hormone levels after surgery. However, nonfunctioning parathyromatosis is more difficult to detect because of the lack of symptoms as well as laboratory abnormalities. Published reports on imaging parathyromatosis are rare. Tublin et al. (1) described 2 patients with parathyromatosis featuring multiple nodules of varying size that were hypoechoic to isoechoic on ultrasonography. They concluded that ultrasonography is an effective tool for the preoperative diagnosis of parathyromatosis and provides information on the location of nodules that aids in successful surgical eradication. Matsuoka et al. (2) suggested that ultrasonography was comparatively the most effective technique for diagnosing parathyromatosis because it is difficult to detect parathyromatosis preoperatively. Fused CT and sestamibi single photon emission CT findings of parathyromoatosis were reported for anatomical correlation of tracer activity (1), but there has been no report of only using CT findings to diagnose parathyromatosis. Contrast-enhanced CT might be more useful than ultrasonography because it can detect the entire extent of parathyromatosis, especially when nodules are located deep beyond the sonic window of ultrasonography. In our case, scattered multiple nodular lesions with lobulated margins appeared in the neck and anterior chest wall along the operative tract on contrast-enhanced dynamic neck CT. These lesions had early homogenous strong enhancement than adjacent soft tissue, including the thyroid gland. Wash-out was shown on the delayed scan. The previously resected parathyroid adenoma, which was presumed to be a thyroid mass, also had a similar washout pattern. As far as we know, there are no imaging reports that compare a nonfunctional parathyroid adenoma with a functional parathyroid adenoma. It was reported that early contrast enhancement and delayed washout on a CT scan are indicative of parathyroid adenoma (7, 8). Secondary parathyromatosis due to a primary parathyroid adenoma is also thought to have similar contrast media dynamics. Early strong enhancement of parathyromatosis may be helpful for differential diagnosis with lymphadenopathy that is morphologically similar to parathyroid adenoma (9).
En bloc resection, including ipsilateral thyroidectomy, was recommended for parathyromatosis, because it is difficult to distinguish between parathyromatosis and parathyroid carcinoma, and due to the possibility of malignancy in previously presenting parathyroid adenoma or hyperplasia (4). Matsuoka et al. (2) routinely performed en bloc resection, including thyroid lobectomy and lymph node dissection as a surgical procedure for their cases. We used the endoscopic axillo-breast approach for the presumed thyroid mass. Endoscopic axillo-breast surgery has a cosmetic advantage due to its remote approach along the fascial plane using an access port, but it is technically difficult and takes more time than open cervical surgery. Various potential complications are brachial plexus injury, Horner syndrome, chyle leakage, and tract or port recurrence (10). In this case, we experienced tumor recurrence after the surgery. Contrast-enhanced dynamic neck CT showed multiple and scattered enhancing nodular lesions with early enhancement and delayed wash-out similar to resected parathyroid adenoma at the operative site and along the endoscopic approach site. To our knowledge, imaging features of nonfunctional parathyromatosis around the operative site and along the tract site after parathyroidectomy have not been reported. This finding supports the hypothesis that parathyromatosis involves spillage and seeding of parathyroid tissue by a capsular tear of the parathyroid adenoma.
Parathyromatosis is rare. We reported imaging findings of nonfunctioning parathyromatosis suggestive of parathyroid tissue seeding by intraoperative parathyroid adenoma capsular injury along the operative site of the endoscopic axillo-breast approach.
Figures and Tables
 | Fig. 1Contrast-enhanced dynamic neck computed tomography showed early enhancing mass (arrow) with delayed wash-out in the posterior aspect of the right thyroid gland. |
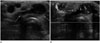 | Fig. 2A 16-year-old girl with parathyromatosis on ultrasonography.
A. Ultrasonography of the anterior neck showed ovoid, homogeneous, and hypoechoic masses (arrows) at the superficial fatty layer of the anterior neck.
B. Color Doppler ultrasonography revealed increased vascularity within the masses.
Note.-Asterisk = vertebral body of cervical spine
|
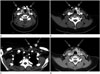 | Fig. 3A 16-year-old girl with parathyromatosis on contrast-enhanced dynamic neck CT.
A-C. Contrast-enhanced dynamic neck computed tomography showed multiple lobulated early enhancing masses (arrows) at the right thyroid lobectomy site, anterior and posterior to strap musculature, and the subcutaneous layer of the anterior neck and left upper chest wall.
D. These (arrows) were similarly dense compared to normal left thyroid lobe on delayed CT scan, measuring 2-15 mm in diameter.
Note.-Asterisk = left thyroid gland
|
References
1. Tublin ME, Yim JH, Carty SE. Recurrent hyperparathyroidism secondary to parathyromatosis: clinical and imaging findings. J Ultrasound Med. 2007; 26:847–851.
2. Matsuoka S, Tominaga Y, Sato T, Uno N, Goto N, Katayama A, et al. Recurrent renal hyperparathyroidism caused by parathyromatosis. World J Surg. 2007; 31:299–305.
3. Meakins JL, Milne CA, Hollomby DJ, Goltzman D. Total parathyroidectomy: parathyroid hormone levels and supernumerary glands in hemodialysis patients. Clin Invest Med. 1984; 7:21–25.
4. Fernandez-Ranvier GG, Khanafshar E, Jensen K, Zarnegar R, Lee J, Kebebew E, et al. Parathyroid carcinoma, atypical parathyroid adenoma, or parathyromatosis? Cancer. 2007; 110:255–264.
5. Palmer JA, Brown WA, Kerr WH, Rosen IB, Watters NA. The surgical aspects of hyperparathyroidism. Arch Surg. 1975; 110:1004–1007.
6. Reddick RL, Costa JC, Marx SJ. Parathyroid hyperplasia and parathyromatosis. Lancet. 1977; 1:549.
7. Rodgers SE, Hunter GJ, Hamberg LM, Schellingerhout D, Doherty DB, Ayers GD, et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery. 2006; 140:932–940. discussion 940-941.
8. Miller DL, Craig WD, Haines GA. Retropharyngeal parathyroid adenoma: precise preoperative localization with CT and arterial infusion of contrast material. AJR Am J Roentgenol. 1997; 169:695–696.
9. Randall GJ, Zald PB, Cohen JI, Hamilton BE. Contrast-enhanced MDCT characteristics of parathyroid adenomas. AJR Am J Roentgenol. 2009; 193:W139–W143.
10. Beninato T, Kleiman DA, Scognamiglio T, Fahey TJ, Zarnegar R. Tract recurrence of a follicular thyroid neoplasm following transaxillary endoscopic thyroidectomy. Thyroid. 2012; 22:214–217.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download