Abstract
Purpose
To determine the prevalence of coronary anomalies using coronary computed tomography angiography (CCTA) and to evaluate the relationship between coronary artery anomalies and chest pain.
Materials and Methods
A total of 12676 patients underwent CCTA scans at our institution between December 2006 and April 2013 using a 64-slice CT and a 128-slice dual-source CT. We determined the prevalence of coronary artery anomalies according to the classification system proposed by Greenberg. The presence or absence of chest pain with each coronary artery anomaly was also evaluated.
Results
Coronary anomalies were found in 176 patients (1.39%) at our institute. Anomalies of origination, course, and termination were detected in 118 (0.93%), 28 (0.22%), and 30 (0.24%) patients, respectively. After the exclusion of 32 patients with combined heart disease, typical (n = 16; 11.1%) or atypical (n = 28; 19.4%) chest pain was present in 44 (30.6%) of the 144 patients at the time of diagnosis.
Anomalies of the coronary arteries are uncommon; the prevalence ranges widely, from 0.285% in autopsies (1) to 5.64% in coronary angiography (CAG) (2). In recent coronary computed tomography angiography (CCTA) studies, the prevalence of coronary artery anomalies ranged from 0.8-5.79% (3, 4, 5, 6). The wide range is caused by the use of various classification systems (2, 3, 7, 8) and patient selection bias according to imaging modality. CCTA has become the apparent standard of reference for the evaluation of coronary artery anomalies with the introduction of the multidetector row CT and the development of an electrocardiography (ECG)-synchronized scanning and reconstruction technique (9, 10, 11, 12, 13, 14). In addition to its non-invasiveness, CCTA was revealed to be superior to conventional angiography in the detection of coronary artery anomalies (15, 16, 17). While most people with coronary anomalies are asymptomatic, recognition of some coronary anomalies, particularly those associated with myocardial ischemia and sudden cardiac death, is important for patient management (18, 19). In this study, we determined the prevalence of coronary anomalies on CCTA and evaluated the relationship between coronary artery anomalies and chest pain at a single center in Korea.
Between December 2006 and April 2013, 13236 CCTA scans were performed out at our institution. After excluding 560 cases of patients who underwent CCTA scans more than twice, 12676 CCTA patient scans were finally enrolled in the present study. The patients' clinical symptoms and cardiovascular risk profiles thickwere obtained through a review of their respective medical charts at the time of the CCTA. We classified patients according to the characteristics of their chest pain as follows: typical, atypical, non-anginal, and no chest pain. According to American College of Cardiology-American Heart Association guidelines (20), typical chest pain refers to 1) substernal chest pain or the ischemic equivalent discomfort, 2) provoked by exertion or emotional stress, and 3) relived by rest or nitroglycerin. Atypical chest pain is defined as chest pain or discomfort with two features of the above three characteristics of typical angina. Non-anginal chest pain is chest pain or discomfort that meets one or none of the typical angina characteristics (21). There was also a no chest pain group. We recorded the presence of other symptoms such as syncope, dyspnea, and palpitation in patients with no chest pain. Hypertension was defined as a systolic blood pressure of 140 mm Hg or greater, diastolic pressure of 90 mm Hg or greater, or current antihypertensive treatment. Diabetes mellitus was defined as a confirmed diagnosis or the use of antidiabetic medication at the time of the study. Smoking was defined as those individuals who currently or previously smoked cigarettes daily. Dyslipidemia was defined as total cholesterol of at least 200 mg/dL or treatment with a lipid-lowering medication. Family history was defined as having a first-degree relative with a documented history of myocardial infarction or sudden cardiac death. The past history included a previous myocardial infarction or stroke. The outcomes were collected through a medical chart review and included coronary artery bypass graft, percutaneous coronary intervention, myocardial infarction, and sudden cardiac death. Our Human Research Committee approved the study design, and informed consent for the present retrospective analysis was waived.
Image acquisition was performed using a Brilliance 64-slice CT scanner (Philips Medical Systems, Eindhoven, the Netherlands) between January 2009 and February 2011 or a dual source 128-slice CT (Somatom Definition FLASH; Siemens Healthcare, Forchheim, Germany) between March 2011 and April 2013.
Retrospective ECG-gated CCTA was performed using a detector collimation of 64 × 0.625 mm, a 400 msec gantry rotation, 216a pitch of 0.2, a tube voltage of 120 kV, and an effective tube current of 600-900 mA with ECG modulation. Thereafter, 80 mL of contrast medium (Iomeron 400; Bracco, Milan, Italy) was intravenously injected at a rate of 4.5 mL/s, followed by a 40 mL saline flush at 4 mL/sec. Scanning was automatically initiated 6 sec after a threshold of 150 Hounsfield units (HU) was achieved in a region of interest in the descending aorta. The 75% R-R interval was primarily used for image reconstruction. In cases of motion artifacts, which reduce the diagnostic quality of images, additional cardiac cycles were explored for images of better quality. Trans-axial images (slice thickness, 0.9 mm; increment, 0.45 mm) were reconstructed using an ECG-gated half-rotation reconstruction algorithm and a sharp kernel (XCB filter).
CCTA was acquired using the retrospective ECG-gating spiral scan protocol and the following parameters: a detector collimation of 128 × 0.6 mm, a 280 msec gantry rotation, a temporal resolution of 75 ms, a tube voltage of 100-120 kV (according to the patient's body mass index), and an effective tube current of 300 mA. The prospective tube current modulation technique was used with a high-dose window of 65-80% of the R-R interval (if heart rate > 75, then the window was between 35% and 50% of the R-R interval) and with the MinDose protocol (Siemens, Forchheim, Germany) in the remaining phases of the cardiac cycle. Contrast material was administered at an injection rate of 4.5 mL/s using a dual-syringe power injector (Stellant D; MedRAD, Indianola, IA, USA). The split-bolus protocol injection was used to inject contrast medium according to body weight. An injection of 60-80 mL of pure, undiluted iodinated contrast material (Iomeron 400; Bracco, Milan, Italy) was followed by a constant volume of 40 mL of a 60%:40% saline-to-contrast medium mixture. Scanning was automatically initiated 7 sec after a threshold of 100 HU was achieved in a region of interest in the ascending aorta. The patient table feed/pitch was variable (range, 0.17-0.38) and was adapted to the heart rate. Retrospective reconstruction was automatically obtained (Best-Phase, Siemens) within in the maximum-dose time window based on the optimal diastolic phase-free motion artifacts. In cases where motion artifacts reduced the diagnostic quality of images, additional cardiac cycles were explored for images of better quality. Images were reconstructed using the following parameters: slice thickness, 0.75 mm; reconstruction increment, 0.4 mm; and convolution kernel, B36f.
Volume rendering technique, maximum intensity projection, multiplanar reformation image analysis, and curve reformation images were conducted using a commercially available workstation (EBW, version 4.5; Philips). An experienced radiologist (D.K.K.) evaluated the CCTA using Vitrea 2 (version 3.8.1; Vital Image, Minnetonka, MN, USA) between December 2006 and February 2011, or using Syngo Via (Siemens Healthcare) between March 2011 and April 2013. We used the classification system proposed by Greenberg et al. (7), who divided coronary artery anomalies into anomalies of origin, anomalies of course, and anomalies of termination. A normal variant is a relatively unusual finding that is observed in > 1% of the same population. An anomaly is a morphologic feature seen in < 1% of the general population (2, 14). The prevalence of myocardial bridging is 15-85% (22) in pathologic analyses and 5.7-30.5% in CCTA (23, 24, 25, 26). Therefore, we classified the myocardial bridge a normal variant and excluded it from the current study. The right coronary artery and conal branch arising separately from the right sinus of Valsalva occur in approximately 50% of the general population (27, 28). Therefore, we classified these anomalies as normal variants and excluded them from the current study.
Microsoft Excel 2007 (Microsoft Corp., Redmond, WA, USA) was used for data collection. We determined the prevalence of coronary artery anomalies according to the classification system proposed by Greenberg et al. (7). The incidence of chest pain with each anomaly was also evaluated. Coronary anomalies considered hemodynamically significant included a single coronary artery, coronary artery origin from the opposite coronary sinus with an interarterial course, and coronary artery fistula (18). The incidence of chest pain in the coronary anomalies considered hemodynamically significant was compared with the other anomalies using the χ2 test. For coronary arteries with an anomalous origin, the interarterial course was divided into high and low interarterial types (29) and the incidence of chest pain was compared using Fisher's exact test. MedCalc (version 12.1.1.0; MedCalc Software, Mariakerke, Belgium) was used for all statistical analyses. All data are expressed as the mean ± standard deviation. p < 0.05 was taken to indicate statistical significance.
Coronary anomalies were found in 176 patients (1.39%) at our institute (Table 1). The mean age of patients was 53 ± 12 years (age range: 19-85 years). Male patients accounted for 69.9% (123/176) of the study population. Anomalies of the origin of the coronary artery were detected in 118 patients (0.93%), including 14 cases of single coronary artery, 31 cases of high takeoff, 13 cases of multiple ostia of the left coronary artery (LCA), 57 cases of origin of a coronary artery from the opposite coronary sinus, and 3 cases of origin of a coronary artery from a non-coronary sinus. Anomalies of the course of the coronary artery were detected in 28 patients (0.22%). Anomalies of coronary artery termination were detected in 30 patients (0.24%), including 25 cases of coronary artery fistula and 5 cases of extracardiac termination. Coronary artery anomalies considered hemodynamically significant were detected in 86 patients (0.68%).
After excluding 32 patients with combined heart disease-14 with obstructive coronary artery disease, 15 with valvular heart disease, and 3 with cardiomyopathy-the clinical symptoms of the remaining 144 patients were evaluated. The clinical symptoms of patients included chest pain (n = 88), dyspnea (n = 18), palpitation (n = 11), and syncope (n = 3). Fifty-six patients had no symptoms (n = 61). Among the 144 patients, 44 (30.6%) had typical (n = 16, 11.1%) or atypical (n = 28, 19.4%) chest pain at the time of diagnosis (Table 2). The coronary anomalies considered hemodynamically significant showed a higher incidence of typical or atypical chest pain than the others [44.9% (31/69) vs. 17.3% (13/75), respectively; p < 0.001]. This finding did not change [21.7% (15/69) vs. 1.3% (1/75), respectively; p < 0.001], when the cases with typical chest pain were selected. Two (22.2%) of nine single coronary artery (Fig. 1), 10 (27.0%) of 37 cases of anomalous origin of a coronary artery with an interarterial course, and 3 (13.0%) of 23 coronary artery fistulas (Fig. 2) had typical chest pain at the time of diagnosis. In cases of anomalous origin of the right coronary artery (RCA) from the left coronary sinus with an interarterial course (n = 44) (Fig. 3), there was no difference in typical chest pain complaints between the high and low interarterial courses [30.8% (8/26) vs. 12.5% (1/8), respectively; p = 0.403].
Table 3 describes the clinical characteristics of 125 patients for whom a clinical cardiovascular risk profile was obtained for the coronary artery anomaly groups with more than five cases. With regard to a past history of cardiovascular disease, three patients [one multiple ostia, one left anterior descending (LAD) duplication, and one coronary artery fistula] had a stroke. The study followed 158 (89.8%) of the 176 patients for a mean of 4.8 ± 1.9 (range 1.5-7.9) years. Of the 39 patients who underwent CAG, two required transcatheter closures of a coronary artery fistula, while the remaining 37 did not require any interventional or surgical treatment of the coronary artery anomaly. Twenty-five patients had a combined coronary artery disease and three had variant angina. Of the four patients who underwent stress nuclear imaging, two patients (one high takeoff, one coronary artery fistula) had ischemia, and the remaining two patients with a termination anomaly had normal findings. There was no myocardial infarction or sudden cardiac death associated with a coronary artery anomaly.
A single coronary artery is an extremely rare congenital anomaly in which only one coronary artery arises with a single ostium from the aortic trunk. The incidence of this anomaly is 0.0024-0.044% according to a database of coronary angiographies (30). The reported incidence was 0.26% in one CCTA study (4). Some of those patients develop typical angina during childhood or adolescence (7). In our study, the prevalence of a single coronary artery was 0.11%, and 22.2% of those patients had typical chest pain. Although most individuals with a single coronary artery have a normal life expectancy, patients are at increased risk of sudden cardiac death if a major coronary branch runs between the pulmonary arteries and aorta (i.e., interarterial course).
Anomalous origins of a coronary artery from the opposite or non-coronary sinus are classified into four recognized patterns. The prevalence in patients who undergo angiography or CCTA was found to be 0.03-0.17% for the RCA arising from the left coronary sinus, 0.09-0.11% for the LCA arising from the right coronary sinus, 0.32-0.67% for the left circumflex artery arising from the right coronary sinus or proximal branch of the RCA, and 0.11% for the LCA or RCA (or a branch of either artery) arising from a non-coronary sinus (6, 31, 32, 33, 34, 35). In this study, the prevalence was 0.45% for coronary arteries with an anomalous origin from the opposite and 0.02% for anomalous origin from the non-coronary sinus. An anomalous origin of the coronary artery from the opposite or non-coronary cusp can take one of the following four courses, depending on the anatomic relationship of the anomalous vessel to the aorta and pulmonary trunk: prepulmonic, interarterial (i.e., a course between the right ventricular outflow tract and aorta), retroaortic, or transseptal (36). Retroaortic, prepulmonic, and transseptal courses seem to be benign, while an interarterial course carries a high risk of myocardial infarction, sudden cardiac death, arrhythmia, and syncope (37). Therefore, the course is important clinically. Compression of the coronary artery between the aorta and pulmonary artery is a possible mechanism for restricted coronary blood flow, particularly during exertion (38, 39, 40, 41, 42). Other possible mechanisms of restricted coronary blood flow are an acute takeoff angle, slit-like ostium, and compression of the intramural segment by the aortic valve commissure (29, 38). In this study, however, only 27.0% of the cases with an interarterial course had typical chest pain. When the interarterial course is further divided into two subtypes; i.e., high and low interarterial courses, Lee et al. (29) reported a higher prevalence of typical angina (43% vs. 6%) in patients with a high interarterial course of anomalous origin of the RCA from the left coronary sinus. However, there was no significant difference (30.8% vs. 12.5%, p = 0.403) in the incidence of typical chest pain between the high and low interarterial courses in our data.
The reported prevalence of myocardial bridges ranges from 15-85% in autopsies and 0.5-2.5% in angiography (22). In a recent report using CCTA, the prevalence was found to be up to 30.5% (23, 24, 25, 26). Therefore, myocardial bridges are considered normal variants rather than anomalies, and we excluded these cases from the present study.
Duplication of the LAD artery has been reported in 0.64-1.3% of the patients undergoing coronary arteriography (31, 32). In a recent ECG-gated cardiac multidetector computed tomography study, the incidence was 0.045% (43). Duplication of the LAD artery usually consists of a short and a long LAD and is categorized into four subtypes (44). In our series, we encountered only type 1 and 4 duplication of the LAD artery. The prevalence was 0.22% and none of the patients had typical chest pain.
Normally, a coronary artery terminates into the capillary bed of the myocardium. A coronary artery fistula is defined as a congenital or acquired coronary artery abnormality in which blood is shunted into a cardiac chamber, the coronary sinus, superior vena cava, pulmonary artery, or pulmonary vein, bypassing the myocardial capillary network (45). It is observed in 0.05-0.25% of patients undergoing diagnostic CAG and 0.2-0.4% of congenital cardiac anomalies (46). The symptoms of coronary artery fistulas mainly depend on the severity of the left-to-right shunt. Myocardial perfusion might be reduced due to the hemodynamic steal phenomenon and this can lead to myocardial ischemia or infarction (47). If symptoms develop, patients present with dyspnea, fatigue, orthopnea, chest pain, endocarditis, arrhythmias, stroke, myocardial ischemia, or myocardial infarction (46). In our series, the prevalence of coronary artery fistula was 0.20%, and 13.0% of the cases had typical chest pain.
The coronary arteries can have connections to extracardiac vessels, such as the bronchial, internal mammary, pericardial, anterior mediastinal, superior and inferior phrenic, and intercostal arteries, as well as the esophageal branch of the aorta (7). Such communications become clinically significant if a condition develops that redirects blood flow from the coronary system to an extra-cardiac vessel because the latter has a lower resistance to flow (48). In this study, the prevalence of extra-cardiac termination was 0.04% and one case had typical chest pain.
This study had several limitations. First, this report was a retrospective single-center study. Therefore, only limited clinical data were available. A multicenter prospective study of the incidence, clinical manifestations, and natural history of each anomaly and their respective treatments will greatly enhance our knowledge of coronary anomalies. Second, the study only considered the relationships between coronary anomalies and chest pain, although some congenital coronary anomalies presented with other symptoms, such as dyspnea, palpitation, or syncope (46). Third, whether the origin of any chest pain was due to a coronary anomaly was not proven in all cases. In this study, however, patients with obstructive coronary artery disease, valvular heart disease, or cardiomyopathy, all of which can lead to chest pain, were excluded. Non-invasive tests including echocardiography or radionuclide myocardial perfusion imaging using either exercise or pharmacologic stress could help to evaluate the cause of the pain.
In conclusion, the prevalence of coronary artery anomalies was 1.39% at our hospital. After excluding patients with combined heart disease, 11.1% had typical chest pain at the time of diagnosis. Knowledge of the appearance of congenital coronary artery anomalies and their clinical significance are important for an accurate diagnosis and will facilitate better patient management in the future.
Figures and Tables
Fig. 1
63 years old male patient with atypical angina.
A, B. Volume rendering technique and three-dimensional maximum intensity projection images show aplasia of right coronary artery (RCA) and collateral vessel from left circumflex artery (arrow).
C. Coronary angiography shows left dominant coronary artery with RCA aplasia.
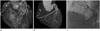
Fig. 2
82 years old male patient with atypical angina.
A, B. Volume rendering technique and coronary angiography show tortuous vessels arising from the proximal left anterior descending (black arrow) and right coronary sinus (white arrow) and draining into pulmonary trunk. Multiple tortuous vascular structures passes anterior to the pulmonary artery and forms a network (*) before it enters the pulmonary trunk.
C. Axial CT image shows the entry site (arrowhead) into pulmonary trunk.

Fig. 3
57 years old female patient with typical angina.
A. Volume rendering technique shows anomalous right coronary artery (RCA) ostium from the left coronary sinus.
B. Oblique sagittal multiplanar reformation image shows RCA (arrow) travels between aorta (Ao) and pulmonary artery (*).
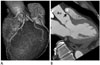
Table 1
Coronary Artery Anomalies Detected in Our Institute (n = 176)

Table 2
Relationship between Coronary Artery Anomalies and Chest Pain (n = 144)

Table 3
Clinical Characteristics of Coronary Artery Anomalies (n = 125)

References
1. Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation. 1956; 14:800–805.
2. Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002; 105:2449–2454.
3. Schmitt R, Froehner S, Brunn J, Wagner M, Brunner H, Cherevatyy O, et al. Congenital anomalies of the coronary arteries: imaging with contrast-enhanced, multidetector computed tomography. Eur Radiol. 2005; 15:1110–1121.
4. Duran C, Kantarci M, Durur Subasi I, Gulbaran M, Sevimli S, Bayram E, et al. Remarkable anatomic anomalies of coronary arteries and their clinical importance: a multidetector computed tomography angiographic study. J Comput Assist Tomogr. 2006; 30:939–948.
5. Srinivasan KG, Gaikwad A, Kannan BR, Ritesh K, Ushanandini KP. Congenital coronary artery anomalies: diagnosis with 64 slice multidetector row computed tomography coronary angiography: a single-centre study. J Med Imaging Radiat Oncol. 2008; 52:148–154.
6. Tariq R, Kureshi SB, Siddiqui UT, Ahmed R. Congenital anomalies of coronary arteries: diagnosis with 64 slice multidetector CT. Eur J Radiol. 2012; 81:1790–1797.
7. Greenberg MA, Fish BG, Spindola-Franco H. Congenital anomalies of the coronary arteries. Classification and significance. Radiol Clin North Am. 1989; 27:1127–1146.
8. Dodge-Khatami A, Mavroudis C, Backer CL. Congenital Heart Surgery Nomenclature and Database Project: anomalies of the coronary arteries. Ann Thorac Surg. 2000; 69:4 Suppl. S270–S297.
9. Kim SY, Seo JB, Do KH, Heo JN, Lee JS, Song JW, et al. Coronary artery anomalies: classification and ECG-gated multi-detector row CT findings with angiographic correlation. Radiographics. 2006; 26:317–333. discussion 333-334.
10. Earls JP. Coronary artery anomalies. Tech Vasc Interv Radiol. 2006; 9:210–217.
11. Kang JW, Seo JB, Chae EJ, Jang YM, Do KH, Lee JS, et al. Coronary artery anomalies: classification and electrocardiogram-gated multidetector computed tomographic findings. Semin Ultrasound CT MR. 2008; 29:182–194.
12. Patel S. Normal and anomalous anatomy of the coronary arteries. Semin Roentgenol. 2008; 43:100–112.
13. Sundaram B, Kreml R, Patel S. Imaging of coronary artery anomalies. Radiol Clin North Am. 2010; 48:711–727.
14. Pursnani A, Jacobs JE, Saremi F, Levisman J, Makaryus AN, Capuñay C, et al. Coronary CTA assessment of coronary anomalies. J Cardiovasc Comput Tomogr. 2012; 6:48–59.
15. van Ooijen PM, Dorgelo J, Zijlstra F, Oudkerk M. Detection, visualization and evaluation of anomalous coronary anatomy on 16-slice multidetector-row CT. Eur Radiol. 2004; 14:2163–2171.
16. Shi H, Aschoff AJ, Brambs HJ, Hoffmann MH. Multislice CT imaging of anomalous coronary arteries. Eur Radiol. 2004; 14:2172–2181.
17. Karaca M, Kirilmaz A, Oncel G, Oncel D, Yilmaz H, Tamci B, et al. Contrast-enhanced 64-slice computed tomography in detection and evaluation of anomalous coronary arteries. Tohoku J Exp Med. 2007; 213:249–259.
18. Shriki JE, Shinbane JS, Rashid MA, Hindoyan A, Withey JG, DeFrance A, et al. Identifying, characterizing, and classifying congenital anomalies of the coronary arteries. Radiographics. 2012; 32:453–468.
19. Tresoldi S, Mezzanzanica M, Campari A, Salerno Uriarte D, Cornalba G. Role of computed tomography coronary angiography in the management of coronary anomalies. J Card Surg. 2013; 28:33–36.
20. Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, et al. ACC/AHA guidelines for exercise testing: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). Circulation. 1997; 96:345–354.
21. Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol. 1983; 1(2 Pt 1):574–575.
22. Möhlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002; 106:2616–2622.
23. Zeina AR, Odeh M, Blinder J, Rosenschein U, Barmeir E. Myocardial bridge: evaluation on MDCT. AJR Am J Roentgenol. 2007; 188:1069–1073.
24. Konen E, Goitein O, Sternik L, Eshet Y, Shemesh J, Di Segni E. The prevalence and anatomical patterns of intramuscular coronary arteries: a coronary computed tomography angiographic study. J Am Coll Cardiol. 2007; 49:587–593.
25. Ko SM, Choi JS, Nam CW, Hur SH. Incidence and clinical significance of myocardial bridging with ECG-gated 16-row MDCT coronary angiography. Int J Cardiovasc Imaging. 2008; 24:445–452.
26. Kim SY, Lee YS, Lee JB, Ryu JK, Choi JY, Chang SG, et al. Evaluation of myocardial bridge with multidetector computed tomography. Circ J. 2010; 74:137–141.
27. Montaudon M, Latrabe V, Iriart X, Caix P, Laurent F. Congenital coronary arteries anomalies: review of the literature and multidetector computed tomography (MDCT)-appearance. Surg Radiol Anat. 2007; 29:343–355.
28. Erol C, Seker M. The prevalence of coronary artery variations on coronary computed tomography angiography. Acta Radiol. 2012; 53:278–284.
29. Lee HJ, Hong YJ, Kim HY, Lee J, Hur J, Choi BW, et al. Anomalous origin of the right coronary artery from the left coronary sinus with an interarterial course: subtypes and clinical importance. Radiology. 2012; 262:101–108.
30. Desmet W, Vanhaecke J, Vrolix M, Van de Werf F, Piessens J, Willems J, et al. Isolated single coronary artery: a review of 50,000 consecutive coronary angiographies. Eur Heart J. 1992; 13:1637–1640.
31. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990; 21:28–40.
32. Kimbiris D, Iskandrian AS, Segal BL, Bemis CE. Anomalous aortic origin of coronary arteries. Circulation. 1978; 58:606–615.
33. Donaldson RM, Raphael M, Radley-Smith R, Yacoub MH, Ross DN. Angiographic identification of primary coronary anomalies causing impaired myocardial perfusion. Cathet Cardiovasc Diagn. 1983; 9:237–249.
34. Chaitman BR, Lespérance J, Saltiel J, Bourassa MG. Clinical, angiographic, and hemodynamic findings in patients with anomalous origin of the coronary arteries. Circulation. 1976; 53:122–131.
35. Bunce NH, Lorenz CH, Keegan J, Lesser J, Reyes EM, Firmin DN, et al. Coronary artery anomalies: assessment with free-breathing three-dimensional coronary MR angiography. Radiology. 2003; 227:201–208.
36. Ropers D, Gehling G, Pohle K, Maeffert R, Regenfus M, Moshage W, et al. Images in cardiovascular medicine. Anomalous course of the left main or left anterior descending coronary artery originating from the right sinus of valsalva: identification of four common variations by electron beam tomography. Circulation. 2002; 105:e42–e43.
37. Cheitlin MD, De Castro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva, A not-so-minor congenital anomaly. Circulation. 1974; 50:780–787.
38. Roberts WC, Siegel RJ, Zipes DP. Origin of the right coronary artery from the left sinus of valsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. 1982; 49:863–868.
39. Virmani R, Chun PK, Goldstein RE, Robinowitz M, McAllister HA. Acute takeoffs of the coronary arteries along the aortic wall and congenital coronary ostial valve-like ridges: association with sudden death. J Am Coll Cardiol. 1984; 3:766–771.
40. Kragel AH, Roberts WC. Anomalous origin of either the right or left main coronary artery from the aorta with subsequent coursing between aorta and pulmonary trunk: analysis of 32 necropsy cases. Am J Cardiol. 1988; 62(10 Pt 1):771–777.
41. Frescura C, Basso C, Thiene G, Corrado D, Pennelli T, Angelini A, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol. 1998; 29:689–695.
42. Lee BY. Anomalous right coronary artery from the left coronary sinus with an interarterial course: is it really dangerous? Korean Circ J. 2009; 39:175–179.
43. Namgung J, Kim JA. The prevalence of coronary anomalies in a single center of Korea: origination, course, and termination anomalies of aberrant coronary arteries detected by ECG-gated cardiac MDCT. BMC Cardiovasc Disord. 2014; 14:48.
44. Spindola-Franco H, Grose R, Solomon N. Dual left anterior descending coronary artery: angiographic description of important variants and surgical implications. Am Heart J. 1983; 105:445–455.
45. Gowda RM, Vasavada BC, Khan IA. Coronary artery fistulas: clinical and therapeutic considerations. Int J Cardiol. 2006; 107:7–10.
46. Zenooz NA, Habibi R, Mammen L, Finn JP, Gilkeson RC. Coronary artery fistulas: CT findings. Radiographics. 2009; 29:781–789.
47. Braden DS, O'Neal KR, McMullan MR, Ebeid MR. Congenital coronary arteriovenous fistula presenting with syncope. Pediatr Cardiol. 2002; 23:218–220.
48. Lee CM, Leung TK, Wang HJ, Lee WH, Shen LK, Chen YY. Identification of a coronary-to-bronchial-artery communication with MDCT shows the diagnostic potential of this new technology: case report and review. J Thorac Imaging. 2007; 22:274–276.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download