This article has been corrected. See "Erratum: Imaging Findings of Epstein-Barr Virus-Associated Gastric Lymphoepithelioma-Like Carcinoma" in Volume 73 on page 66.
Abstract
Purpose
To analyze the computed tomography (CT) features of Epstein-Barr virus (EBV)-associated lymphoepithelioma-like carcinoma (LELC).
Materials and Methods
Between January 2004 and September 2014, radiologic features of 44 EBV-associated LELCs were analyzed. Lesion detectability, multiplicity, location, gross appearance, lesion thickness and margin, presence of a round edge, contrast enhancement pattern, and the degree of contrast enhancement were analyzed.
Results
The most common location of LELC was the upper third of the stomach (n = 28; 63.64%). A high percentage of cases showed uniform peripheral thickness (n = 32; 88.89%). LELCs demonstrated well-defined margins in a high percentage of cases (n = 31; 86.11%). Additionally, a high percentage of cases showed the presence of a round edge (n = 28; 77.78%).
Epstein-Barr virus (EBV) affects the pathogenesis of various tumors. EBV may play an oncogenic role in nasopharyngeal carcinoma, Burkitt's lymphoma, sinonasal T-cell lymphoma, B-cell lymphoma, and Hodgkin's lymphoma (1, 2). Primary gastric lymphoepithelioma-like carcinoma (LELC) resembles the histologic pattern of nasopharyngeal cancer. It is reported that over 80% to 90% of gastric LELC demonstrate EBV infections (3). LELC of the stomach is a rare type of undifferentiated carcinoma with an intense lymphoplasmacytoid stroma, which was first described in 1976 (4). Histologically, primary gastric LELC demonstrates moderate-poorly differentiation with prominent intratumoral lymphocytic infiltration (5, 6). Patients with EBV-associated LELC have a better prognosis than those with conventional gastric adenocarcinoma (7). The incidence of this tumor is less common than the conventional gastric adenocarcinoma. However, this lesion reportedly constitutes 7% to 16% of all gastric adenocarcinomas (8, 9).
Sporadic reports have described several characteristics of LELC. Most of these studies evaluated the clinicopathologic features of LELC (7, 10). However, to date, there have been few studies about the computed tomography (CT) features of EBV-associated LELC (11). The purpose of this study was to analyze the clinical and radiological features of EBV-associated LELC and to determine which CT features are seen more often in EBV-associated LELC.
This retrospective study was approved by our Institutional Review Board, which waived the requirement to obtain informed consent. Between January 2004 and September 2014, EBV in situ hybridization (ISH) tests were performed on surgical specimens of 123 gastric carcinoma patients, many of which showed variable lymphocytic infiltration, which may suggest the possibility of LELC. Eighty-four patient specimens that demonstrated EBV positivity on the EBV-ISH test were initially included as EBV-associated LELC. Of these specimens, 40 were excluded from analysis because they showed pathologic features of conventional gastric adenocarcinoma, neuroendocrine tumor, or signet-ring cell carcinoma. The surgical specimens of the remaining 44 patients fulfilled the pathologic criterion for LELC with EBV positivity. The tumors in 39 patients that demonstrated EBV negativity on EBV-ISH test were also excluded. A total of 44 patients received esophagogastroduodenoscopy (EGD) before surgery or endoscopic submucosal dissection (ESD). The interval between EGD and CT was less than 2 weeks. All patients underwent surgery or ESD. Patients underwent total gastrectomy (n = 21; 47.73%), subtotal gastrectomy (n = 15; 34.09%), proximal gastrectomy (n = 4; 9.09%), or ESD (n = 4; 9.09%).
All patients underwent CT examinations with one of four commercially available 16-, 64-, or 128-channel multidetector CT (MDCT) scanners: Somatom Definition flash, Somatom Sensation 16 (Siemens Medical Solutions, Erlangen, Germany), Briliance 16, and Briliance 64 (Philips Healthcare, Cleveland, OH, USA), respectively.
All patients fasted for over 6 hours and gave written consent before undergoing the CT scan. They did not receive intravenous anticholinergic drugs or glucagon injections beforehand. Patients underwent CT scanning immediately after drinking 700-1000 mL of pure water for adequate stomach distension, in a routine supine position to avoid artifacts caused by air in the stomach. An unenhanced CT was taken first, and for contrast-enhanced CT, 150 cc of nonionic contrast agent (Ioversol, Optiray; Mallinckrodt Pharmaceuticals, Dublin, Ireland) was administrated intravenously at rate of 3 mL/sec using a power injector. The second CT scan was performed 30 seconds after the administration of the contrast agent to obtain the arterial phase. The third CT scan was performed 90 seconds after the contrast agent was administered to obtain the portal venous phase. The scanning ranged from the xyphoid process to the lower end of the symphysis pubis in the portal venous phase; for the unenhanced and arterial phase enhanced CTs, the scanning range was from the xyphoid process to the level including the whole stomach.
CT scanning parameters were as follows: for 16 detector rows, a beam collimation of 1.5 × 16 mm, a pitch of 0.938, kVp/mA 120/300, and a gantry rotation time of 0.75 seconds; for 64 detector rows, a beam collimation of 0.625 × 64 mm, a pitch of 0.891, kVp/mA 120/300, and a gantry rotation time of 0.75 seconds; and for 128 detector rows, a beam collimation of 0.6 × 128 mm, a pitch of 0.6, kVp/mA 120/300, and a gantry rotation time of 0.5 seconds. Isotropic raw data was acquired with a slice thickness of 1 mm and an interval of 1 mm using MDCT. Using these raw data, transverse images were obtained with a slice thickness of 5 mm and an interval of 5 mm; the coronal maximum intensity projection (MIP) images were reconstructed on a workstation with arterial phase contrast CT scans, and coronal and sagittal multiplanar reformation (MPR) images were reconstructed on the workstation with portal venous phase contrast CT scans. Each MIP image was obtained at a 30 mm interval with a slice thickness of 3 mm, and each MPR image was obtained at a 3 mm interval with a slice thickness of 3 mm.
All CT images were analyzed retrospectively by two radiologists in consensus (with 7 and 12 years of experience, respectively). The qualities of all CT images were adequate for image analysis without artifacts that could hinder correct image evaluation. The reviewers evaluated tumor detectability on CT images with the aid of two-dimensional reformatted images (coronal and sagittal). Second, the number of tumors was recorded to evaluate multiplicity (single or multiple). Third, the following items were analyzed: tumor location, long diameter of tumor, gross appearance, tumor thickness, tumor margin, presence of round edge, contrast enhancement pattern, and degree of contrast enhancement (12). The location of lesions was classified as upper, middle, or lower third of the stomach. The long diameter was measured in mm with an electronic caliper on CT images. The gross appearance of each mass lesion was categorized as 1) polypoid, defined as an intraluminal growing mass; 2) fungating, defined as a lesion with a focal wall thickening of more than 1 cm, with or without a depressed area; 3) ulcerated, defined as a depressed lesion with a wall thickening of less than 1 cm; or 4) diffusely infiltrative, defined as a lesion involving more than 50% of the entire stomach wall (13). As another morphologic criterion, the peripheral thickness of the mass was recorded as uniform or variable (14). When the periphery of a mass had a uniform thickness of within 10% along the horizontal cross-section in CT scans, the peripheral thickness of tumor was considered as uniform (Fig. 1). When a tumor had a well-defined margin, the reviewers recorded presence or absence of a round edge on the CT scans. The tumor location, multiplicity, long diameter of tumor, gross appearance, the presence of uniform peripheral thickness, and well-defined margin were correlated with histologic specimens that were confirmed by a pathologist. The contrast enhancements of the tumors were classified as of a homogenous or heterogeneous pattern. The degree of enhancement was classified as high enhancement (tumor attenuation was higher than that of adjacent normal gastric mucosa), moderate enhancement (tumor attenuation was comparable with that of gastric mucosa), and low enhancement (tumor attenuation was lower than that of gastric mucosa) (15).
All surgical specimens were evaluated by one experienced pathologist (12 years of experience). The criteria of EBV-associated LELC were defined as follows: 1) well-defined tumor margin, 2) dense lymphocytic infiltration of a degree whereby tumor infiltrating lymphocytes outnumbered the tumor cells, 3) syncytial growth pattern and/or poorly formed glandular structures, and 4) presence of EBV in gastric tumor cells as determined by EBV-encoded RNA (EBER) in situ hybridization. On the other hand, cases showing scattered lymphocytes with prominent desmoplasia and only one or two lymphoid aggregates per tissue section were classified as conventional adenocarcinoma. All these cases were determined to be EBER-negative. Cases that showed both LELC areas and conventional adenocarcinomatous area were excluded in this analysis. The presence or absence of round edge was confirmed by reviewing hematoxylin and eosin (H&E)-stained slides of surgical specimens. Finally, according to the 7th American Joint Committee on Cancer (AJCC) TNM staging system, the pathological TNM stage was assessed.
The mean age of patients with EBV-associated LELC was 58.18 years old (range: 34-82 years old). Male predominance (male to female ratio = 35:9) was seen in patients with EBV-associated LELC. Multiple lesions were detected in only three patients with EBV-associated LELCs. The most common location of EBV-associated LELC was the upper third of the stomach (n = 28; 63.64%).
According to the 7th AJCC classification, TNM stages of EBV-associated LELCs were T1aN0M0 (n = 1), T1bN0M0 (n = 21), T1bN1M0 (n = 3), T2N0M0 (n = 5), T2N1M0 (n = 2), T2N2M0 (n = 1), T3N0M0 (n = 6), T3N1M0 (n = 1), T3N2M0 (n = 1), T3N3M0 (n = 1), T4aN0M0 (n = 1), and T4aN3M0 (n = 1). Lymph node metastasis was seen in 10 cases (22.73%) of EBV-associated LELCs. The clinical characteristics of EBV-positive LELC are summarized in Table 1.
Detectability of the tumors was 81.82% (n = 36) in EBV-associated LELCs. Eight cases of EBV-associated LELCs were not detected on CT scans. The long diameters of EBV-associated LELCs ranged from 6 mm to 90 mm (mean, 25.83 mm).
Fungating lesions were the most common type of gross appearance; 24 patients (66.67%) presented with them (Fig. 2). The second most common type was ulcerative lesion, which was seen in 9 patients (25%) (Fig. 3). The polypoid appearance was demonstrated in two cases of 44 EBV-associated LELCs (Fig. 4). The infiltrative lesion was the least common type of gross appearance, as only one patient showed that type (2.78%).
A high percentage of cases showed the presence of uniform peripheral thickness in EBV-associated LELCs; 32 patients (88.89%). EBV-associated LELCs demonstrated well-defined margin (n = 31; 86.11%) in more cases. A high percentage of cases showed the presence of round edge; 28 patients (77.78%).
The most common pattern of contrast enhancement was the homogeneous type as shown in Table 2 (n = 32; 88.89%). Four cases of EBV-associated LELC showed a heterogeneous pattern of contrast enhancement. The most common degree of contrast enhancement was moderate; 25 patients (69.44%). The second most common degree was high; nine patients (25%) with EBV-associated LELCs (Fig. 5). The low degree of contrast enhancement were seen in just two patients (5.56%).
EBV-associated LELCs developed in male patients (n = 35) more often than in female patients (n = 9). The results support a previous meta-analysis (3): the incidence of EBV-associated LELC was four times higher in males than females but that of conventional gastric adenocarcinoma was twice as high in males than in females. The mean age of patients with EBV-associated LELC was 58.18 years old (range: 34-82 years old). To date, the prevalent age of EBV-associated LELCs are controversial (16, 17). The peak prevalence of conventional adenocarcinoma is known to be between 50 and 70 years old (18). Thus, this may suggest little age difference between the two groups when compared with our study results.
Multiple lesions were detected only in three patients with EBV-associated LELCs. Previous researchers reported that EBV-associated LELCs tended to be highly associated with multiplicity (9, 16). But, in our study, multiplicity of LELC was seen in only few cases. Lymph node metastasis was also seen in a small percentage of cases (n = 10; 22.73%). According to previous reports, EBV-associated LELC showed association with significantly lower T stage and lower N stage as compared with conventional adenocarcinoma (19, 20). One study showed 37% (n = 46/123) of N0 cancers in EBV-associated LELC group compared with 6.4% (n = 26/405) of N0 cancers in the conventional gastric carcinoma group (19).
Detectability of the tumors was 81.82% (n = 36) in our study. In previous reports, the detection rate of gastric cancer using MDCT ranged from 86.5% to 100% in advanced cases and 32% to 96.7% in early cases (21, 22, 23).
In our CT imaging analysis, the most common location of EBV-associated LELC was the upper third of the stomach (63.64%). The results of our research agree with previous reports that EBV-associated LELCs occur more frequently in the upper third of the stomach (1, 17, 24, 25). As for conventional adenocarcinomas, they were reported to be located more often in either middle or lower third of stomach (19).
The most common type of gross appearance was the fungating type (n = 24; 66.67%). The second most common type of gross appearance was the ulcerative type (n = 9; 25%). The polypoid appearance was demonstrated only in two of the 44 EBV-associated LELCs. The infiltrative lesion was the least common type of gross appearance. The results of our study support the previous research (11) which found that EBV-associated LELCs show as bulging masses from gastric wall. In one large series, the percentage of each subtype of conventional advanced gastric carcinoma was polypoid in 4.7%; fungating in 17.7%; ulcerative in 66.6%; and infiltrative in 11% (26). Compared with this previous result, a higher percentage of the fungating type was seen in our LELC study.
A high percentage of cases showed the presence of uniform peripheral thickness of the tumors in EBV-associated LELCs (n = 32; 88.89%). EBV-associated LELCs demonstrated well-defined margins in 31 cases (86.11%). A high percentage of cases showed the presence of round edges in EBV-associated LELCs (n = 28; 77.78%). EBV-associated LELC reportedly demonstrated expansive growth of nodules with submucosal invasion in the stomach wall (17, 27). Watanabe et al. (4) reported that, at the periphery of the tumor, the lymphoid cells infiltrated the tissue of gastric wall immediately beyond the advancing margin of carcinoma cells. But, as a rule, the margins of the stromal infiltrate as well as that of the tumor cells were sharply delineated against the surrounding stomach wall. In addition, several researchers reported that the tumor cells, which are arranged in sheets or nests, are surrounded by a dense lymphocytic infiltration (4, 17, 28). These pathological features may contribute to the round uniform edges of LELCs.
The uniform peripheral thickness with round edge that was more commonly found in EBV-associated LELCs can be described as having uniform thickness profile that may resemble a pizza crust edge. Presently, CT features of the LELC are not familiar to clinicians. As EBV-associated LELCs have a low risk of lymph node metastasis and better prognosis, differentiation of LELCs from conventional adenocarcinomas may allow tailored therapeutic management in such patients in the future, such as medical treatment with a demethylation agent that may induce lysis of EBV infected cancer cells (7, 18, 20, 29). Understanding the imaging findings of EBV-associated gastric carcinoma is important in differentiating it from other tumors, such as submucosal tumor and poorly differentiated adenocarcinoma (30). In a case where the pathological features are similar to poorly differentiated conventional adenocarcinoma, specific imaging features of EBV-associated LELC can help clinicians to decide whether additional tests such as EBV-ISH are necessary (19). A recent study (19) including 123 EBV-associated gastric carcinoma cases showed that 18 (14.6%) cases had histologic features that were pathologically indistinguishable from conventional adenocarcinoma in terms of H&E staining.
The most common pattern of contrast enhancement was the homogeneous type (n = 32; 88.89%) and the most common degree of contrast enhancement was moderate (n = 25; 69.44%). Park et al. (13) reported that the most common enhancement pattern and grade of conventional gastric adenocarcinoma were a homogenous pattern and high enhancement grade. However, to date, there is no report about the patterns and grade of EBV-associated LELCs.
There are some limitations in our study. First, our study was a retrospective study, which could have inherent selection bias. Second, our study involves a small sample size because there were a few patients who had undergone EBV-ISH tests. LELC constitutes a small percentage of all gastric adenocarcinomas and EBV-ISH tests were performed selectively by pathologists when EBV-associated gastric carcinoma was pathologically suspected. Widely available and relatively accurate EBV-ISH (8) was used as a standard value and untested cases were not included as some EBV-associated gastric carcinomas may show similar pathologic features as conventional adenocarcinomas (19). To avoid bias in our study, EBV-ISH negative LELCs and EBV-positive conventional adenocarcinomas were excluded.
In conclusion, when a patient shows radiologic features, including a tumor location in the upper third of the stomach, and presence of a uniform thickness profile of the mass with a round edge resembling a pizza crust, such features may suggest the possibility of EBV-associated LELC.
Figures and Tables
 | Fig. 1Technique for peripheral thickness measurement of a mass. Thickness of a mass was measured at the peripheral portion. When (a) and (b) show less than 10% difference in length, it is defined as positive presence of uniform thickness profile. In addition to uniform thickness profile, round edges (arrows) were seen in the masses resembling pizza crust. |
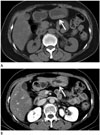 | Fig. 2A 58-year-old female with Epstein-Barr virus-associated lymphoepithelioma-like carcinoma in the stomach. Precontrast (A) and venous phase contrast-enhanced (B) axial CT scans show fungating mass with round edge and moderate enhancement (arrows) in the antrum of stomach. |
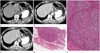 | Fig. 3A 63-year-old male with Epstein-Barr virus-associated lymphoepithelioma-like carcinoma in the stomach. Precontrast- (A), arterial phase contrast- (B) and venous phase contrast- (C) enhanced axial CT scans show ulcerative mass with uniform peripheral thickness, round edge and homogeneous enhancement (arrows) in the upper third of stomach. D. Low-power photomicrograph (hematoxylin & eosin stain, × 4) shows well-demarcated mass of lymphoid stroma with uniform thickness and round edge at both sides of tumor. E. High-power photomicrograph (hematoxylin & eosin stain, × 40) shows uniformly distributed multifocal nests of poorly differentiated adenocarcinomas in the lymphoid stroma. |
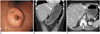 | Fig. 4A 54-year-old female with Epstein-Barr virus-associated lymphoepithelioma-like carcinoma in the stomach. A. Esophageal gastroduodenoscopy revealed submucosal mass with mucosal ulcerations in the upper third of stomach. Contrast-enhanced coronal multiplanar reconstruction (B) and contrast-enhanced axial CT scan (C) show small polypoid mass with round edge and low enhancement (arrows). |
 | Fig. 5A 69-year-old male with Epstein-Barr virus-associated lymphoepithelioma-like carcinoma in the stomach. Arterial phase (A) and venous phase (B) contrast-enhanced axial CT scans show fungating mass with round edge, uniform peripheral thickness, and high enhancement (arrows) in the lower third of stomach. C. Low-power photomicrograph (hematoxylin & eosin stain, × 4) shows well-demarcated mass of lymphoid stroma. The mass shows uniform peripheral thickness with round well-demarcated edge. Reactive lymphoid follicles (open arrows) are scattered between tumor nests with dense lymphocytic infiltration. |
Table 1
Clinical and Pathological Characteristics of Patients with EBV-Associated LELCs

Table 2
CT Features of EBV-Associated LELCs

References
1. Kijima Y, Hokita S, Takao S, Baba M, Natsugoe S, Yoshinaka H, et al. Epstein-Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J Med Virol. 2001; 64:513–518.
2. Selves J, Bibeau F, Brousset P, Meggetto F, Mazerolles C, Voigt JJ, et al. Epstein-Barr virus latent and replicative gene expression in gastric carcinoma. Histopathology. 1996; 28:121–127.
3. Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009; 137:824–833.
4. Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976; 38:232–243.
5. Ushiku T, Shinozaki A, Uozaki H, Iwasaki Y, Tateishi Y, Funata N, et al. Gastric carcinoma with osteoclast-like giant cells. Lymphoepithelioma-like carcinoma with Epstein-Barr virus infection is the predominant type. Pathol Int. 2010; 60:551–558.
6. Torlakovic G, Snover DC, Torlakovic E. Simultaneous EBV-positive lymphoepithelioma-like carcinoma and EBV-negative intestinal-type adenocarcinoma in a patient with Helicobacter pylori-associated chronic gastritis. Am J Clin Pathol. 2004; 121:237–243.
7. Herath CH, Chetty R. Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma. Arch Pathol Lab Med. 2008; 132:706–709.
8. Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992; 140:769–774.
9. Fukayama M, Chong JM, Uozaki H. Pathology and molecular pathology of Epstein-Barr virus-associated gastric carcinoma. Curr Top Microbiol Immunol. 2001; 258:91–102.
10. Wang HH, Wu MS, Shun CT, Wang HP, Lin CC, Lin JT. Lymphoepithelioma-like carcinoma of the stomach: a subset of gastric carcinoma with distinct clinicopathological features and high prevalence of Epstein-Barr virus infection. Hepatogastroenterology. 1999; 46:1214–1219.
11. Maeda E, Akahane M, Uozaki H, Kato N, Hayashi N, Fukayama M, et al. CT appearance of Epstein-Barr virus-associated gastric carcinoma. Abdom Imaging. 2009; 34:618–625.
12. Kim JY, Lee JM, Kim KW, Park HS, Choi JY, Kim SH, et al. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology. 2009; 252:92–100.
13. Park MS, Yu JS, Kim MJ, Yoon SW, Kim SH, Noh TW, et al. Mucinous versus nonmucinous gastric carcinoma: differentiation with helical CT. Radiology. 2002; 223:540–546.
14. Balthazar EJ. CT of the gastrointestinal tract: principles and interpretation. AJR Am J Roentgenol. 1991; 156:23–32.
15. Hur BY, Kim SH, Choi JY, Rha SE, Lee MW, Kim SY, et al. Gastroduodenal glomus tumors: differentiation from other subepithelial lesions based on dynamic contrast-enhanced CT findings. AJR Am J Roentgenol. 2011; 197:1351–1359.
16. Tokunaga M, Land CE, Uemura Y, Tokudome T, Tanaka S, Sato E. Epstein-Barr virus in gastric carcinoma. Am J Pathol. 1993; 143:1250–1254.
17. Uozaki H, Fukayama M. Epstein-Barr virus and gastric carcinoma--viral carcinogenesis through epigenetic mechanisms. Int J Clin Exp Pathol. 2008; 1:198–216.
18. Kim JH, Eun HW, Goo DE, Shim CS, Auh YH. Imaging of various gastric lesions with 2D MPR and CT gastrography performed with multidetector CT. Radiographics. 2006; 26:1101–1116. discussion 1117-1118.
19. Song HJ, Srivastava A, Lee J, Kim YS, Kim KM, Ki Kang W, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010; 139:84–92.
20. van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004; 22:664–670.
21. Moschetta M, Stabile Ianora AA, Anglani A, Marzullo A, Scardapane A, Angelelli G. Preoperative T staging of gastric carcinoma obtained by MDCT vessel probe reconstructions and correlations with histological findings. Eur Radiol. 2010; 20:138–145.
22. Hwang SW, Lee DH, Lee SH, Park YS, Hwang JH, Kim JW, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol. 2010; 25:512–518.
23. Bhandari S, Shim CS, Kim JH, Jung IS, Cho JY, Lee JS, et al. Usefulness of three-dimensional, multidetector row CT (virtual gastroscopy and multiplanar reconstruction) in the evaluation of gastric cancer: a comparison with conventional endoscopy, EUS, and histopathology. Gastrointest Endosc. 2004; 59:619–626.
24. Yanai H, Nishikawa J, Mizugaki Y, Shimizu N, Takada K, Matsusaki K, et al. Endoscopic and pathologic features of Epstein-Barr virus-associated gastric carcinoma. Endosc. 1997; 45:236–242.
25. Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT, Chang MC, et al. Epstein-Barr virus-associated gastric carcinomas: relation to H. pylori infection and genetic alterations. Gastroenterology. 2000; 118:1031–1038.
26. Kim DY, Kim HR, Kim YJ, Kim S. Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg. 2002; 72:739–742.
27. Nishikawa J, Yanai H, Mizugaki Y, Takada K, Tada M, Okita K. Case report: hypoechoic submucosal nodules: a sign of Epstein-Barr virus-associated early gastric cancer. J Gastroenterol Hepatol. 1998; 13:585–590.
28. Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995; 103:308–315.
29. Feng WH, Hong G, Delecluse HJ, Kenney SC. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol. 2004; 78:1893–1902.
30. Kim SW, Shin HC, Kim IY, Kim CJ, Lee JH, Lee CK, et al. Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma presenting as a submucosal mass: CT findings with pathologic correlation. Korean J Radiol. 2010; 11:697–700.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download