Abstract
Pulmonary artery dissection is rarer than other intraluminal diseases of pulmonary artery such as pulmonary thromboembolism or pulmonary artery sarcoma. We report two cases of pulmonary artery dissection mimicking pulmonary artery sarcoma. Computed tomography (CT) showed no enhancement of intrapulmonary arterial lesion or expansion of involved pulmonary artery. Magnetic resonance imaging (MRI) showed low-signal intensity intimal flap on T1- and T2-weighted images. There was no fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET)-CT. In this case report, we describe the imaging features of pulmonary artery dissection on CT, MRI, and PET-CT.
Pulmonary artery dissection is a disease causing high mortality. Over the past two centuries, approximately 50 cases with pulmonary artery dissection have been reported in the literature, including eight diagnosed while patients were alive (1). Symptoms of pulmonary artery dissection are variable. It may start with acute onset of chest pain, dyspnea, and hemoptysis (2, 3, 4). It may initially present with only ambiguous symptoms, such as respiration-associated pain or distress (5). These nonspecific symptoms can be caused by pulmonary artery sarcoma or pulmonary embolism shown as intraluminal pulmonary artery lesion on imaging studies. Several radiologic findings that we present in this case report can help the differentiation of pulmonary artery dissection from other mimickers.
A 48-year-old woman presented to our hospital with dry cough and dyspnea for two weeks. She had no remarkable past medical history. She didn't complain of obvious chest pain. Chest radiography and echocardiography showed no abnormality in heart or lungs. Contrast-enhanced chest computed tomography (CT) scans showed a smooth surfaced, homogeneous, and slightly low-attenuation filling defect within the lumen of left main pulmonary artery extending to the left lower lobar artery (Fig. 1A). There was no luminal dilatation compared to contralateral pulmonary artery. Magnetic resonance imaging (MRI) was performed to distinguish the lesion from thrombosis and solid tumors such as pulmonary artery sarcoma. T1- and T2-weighted images showed an intraluminal lesion with the same bright signal intensity as subcutaneous fat layer covered by low-signal intensity line (Fig. 1B, C).
A 68-year-old woman with a 7-day history of medical treatment for dry cough, general ache, and dyspnea in a local hospital was referred to our Cardiology Department for further evaluation. Echocardiography and treadmill test done in local hospital showed unremarkable findings. Initial chest radiograph and laboratory study were normal. Contrast enhanced chest CT scan revealed a smooth surfaced and homogeneous density lesion within the lumen of left main pulmonary artery extending to the left lower lobar artery. The lesion occupied the entire lumen of the pulmonary artery without luminal dilatation. Compared to contralateral pulmonary artery, the lesion was not enhanced (Fig. 2A-C). Positron emission tomography (PET)-CT showed no demonstrable increased fluorodeoxyglucose (FDG) uptake (Fig. 2D).
For the treatment and confirmation of the diagnosis, pneumonectomy was done for these patients. Gross cross section of pulmonary artery showed hematoma in the lumen (Fig. 3A). Microscopic examination revealed that the smooth muscle layer was dissected (Fig. 3B-D). These patients were confirmed as pulmonary artery dissection.
Pulmonary artery dissection is a highly life-threatening disease. Unlike aortic dissection, the false lumen in pulmonary artery dissection tends to rupture rather than to develop a re-entry site. It is frequently followed by sudden death as the dissection transects into the pericardium, causing acute cardiac tamponade (2).
The majority of pulmonary artery dissections occur in the presence of medial degeneration with fragmentation of elastic fibers and generalized dilatation of the pulmonary arterial tree caused by chronic pulmonary hypertension (1). Less common causes of pulmonary artery dissection include chronic inflammation of the pulmonary arteries, right sided endocarditis, amyloidosis, trauma, and sever atherosclerosis.
Due to its high mortality rate, a definitive diagnosis of pulmonary artery dissection is almost always made by autopsy. There are a few literatures referring to the treatment of pulmonary artery dissection. Although the guideline for treatment has not been clearly established, surgery remains the only curative option for patients with pulmonary artery dissection (4). However, its prognosis remains unclear.
Pulmonary thromboembolism, pulmonary artery sarcoma, and pulmonary artery invasion by lung cancer may be observed as intraluminal diseases of pulmonary artery in radiologic studies. Pulmonary artery invasion by lung cancer is almost always accompanied by pulmonary lesion, which is a distinguishable attribute from pulmonary artery dissection. On the other hand, pulmonary thromboembolism and pulmonary artery sarcoma may present as isolated intraluminal lesion of pulmonary artery on radiologic studies, mimicking pulmonary artery dissection.
Mohammad et al. (1) and Neimatallah et al. (2) reported that contrast enhanced chest CT scan in the case of pulmonary artery dissection showed a linear low-density within involved pulmonary artery, which was intimal dissection flap. Similar to CT findings, MRI of pulmonary artery dissection showed intimal dissection flap and low-signal intensity similar to pulmonary artery wall (1, 3). In the two cases that we presented, there was no linear low-density lesion to suggest intimal flap on contrast enhanced chest CT scan. But in the first case, MRI demonstrated hyperintense intraluminal lesion covered by low-signal intensity rim corresponding to hematoma covered by intimal flap in the pathologic study. Low-signal intensity intimal flap on MRI can be a useful finding of pulmonary artery dissection for diagnosis.
Hematoma in late subacute stage (7 days to 30 days) is usually shown as high signal intensity on T1- and T2-weighted images (6), corresponding to the two weeks history in our first case. Pulmonary artery sarcoma is described as minimally higher signal intensity compared to that of chest wall muscle on T1- and T2-weighted images (7). According to the duration, hematoma can be represented with variable signal intensities on MRI. Therefore, correlation with clinical history is required for differentiating hematoma from pulmonary artery sarcoma.
CT findings of pulmonary artery sarcoma are filling defect occupying the entire lumen of the main or proximal pulmonary arteries, expansion of any portion of the involved pulmonary artery, extraluminal extension, and heterogeneous enhancement (8). In the two cases that we presented, no enhancement or expansion of involved pulmonary artery was the important point in differentiating pulmonary artery dissection from pulmonary artery sarcoma. The standardized uptake values on FDG PET is useful to distinguish a benign tumor from a malignant tumor (9). Several case reports have revealed pulmonary artery sarcoma showed FDG uptake within the pulmonary artery on integrated PET-CT, whereas blood thrombi showed no FDG uptake (9, 10). In our second case, there was no FDG uptake on PET-CT.
In this case report, we described imaging features to differentiate pulmonary artery dissection from other intraluminal diseases of pulmonary artery based on the findings of CT, MRI, and PET-CT. Although pulmonary artery dissection is a rare disease, its imaging features are valuable for differential diagnosis of intraluminal disease of pulmonary artery.
Figures and Tables
 | Fig. 1Contrast-enhanced axial CT (A) shows homogenous low density lesion (star) in left main pulmonary artery that occupies the entire lumen of the pulmonary artery. T1- (B) and T2- (C) weighted images show a relatively homogeneous high signal intensity lesion (star), covered by low-signal intensity line (arrow) (case 1). |
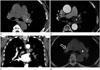 | Fig. 2Non-enhanced axial CT (A) and contrast-enhanced axial CT (B) show no enhancement of left main pulmonary arterial lesion (star). Contrast enhanced coronal CT (C) shows that the lesion (star) extends to left lower lobar artery (arrow). Positron emission tomography-CT (D) shows negative fluorodeoxyglucose (FDG) uptake (star). Calcified right perihilar lymph nodes (open arrow) show mild FDG uptake (case 2). |
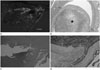 | Fig. 368-year-old woman patient's gross specimen (A) shows hematoma in the pulmonary artery (arrows). Microscopic examination (hematoxylin and eosin stain, × 20) (B) reveals that the hematoma (star) is located in dissected smooth muscle layer (open arrows). 48-year-old woman patient's hematoxylin and eosin stain (× 20) (C) and elastic stain (× 20) (D) of specimen show that the smooth muscle of the pulmonary artery split into two layers (arrows). A, B. Case 2. C, D. Case 1. |
References
1. Mohammad K, Sahlol M, Egiebor O, Sadikot RT. Idiopathic pulmonary artery dissection: a case report. J Med Case Rep. 2009; 3:7426.
2. Neimatallah MA, Hassan W, Moursi M, Al Kadhi Y. CT findings of pulmonary artery dissection. Br J Radiol. 2007; 80:e61–e63.
3. Stern EJ, Graham C, Gamsu G, Golden JA, Higgins CB. Pulmonary artery dissection: MR findings. J Comput Assist Tomogr. 1992; 16:481–483.
4. Degano B, Prevot G, Têtu L, Sitbon O, Simonneau G, Humbert M. Fatal dissection of the pulmonary artery in pulmonary arterial hypertension. Eur Respir Rev. 2009; 18:181–185.
5. Wunderbaldinger P, Bernhard C, Uffmann M, Kürkciyan I, Senbaklavaci O, Herold CJ. Acute pulmonary trunk dissection in a patient with primary pulmonary hypertension. J Comput Assist Tomogr. 2000; 24:92–95.
6. Allkemper T, Tombach B, Schwindt W, Kugel H, Schilling M, Debus O, et al. Acute and subacute intracerebral hemorrhages: comparison of MR imaging at 1.5 and 3.0 T--initial experience. Radiology. 2004; 232:874–881.
7. Bressler EL, Nelson JM. Primary pulmonary artery sarcoma: diagnosis with CT, MR imaging, and transthoracic needle biopsy. AJR Am J Roentgenol. 1992; 159:702–704.
8. Yi CA, Lee KS, Choe YH, Han D, Kwon OJ, Kim S. Computed tomography in pulmonary artery sarcoma: distinguishing features from pulmonary embolic disease. J Comput Assist Tomogr. 2004; 28:34–39.
9. Chong S, Kim TS, Kim BT, Cho EY, Kim J. Pulmonary artery sarcoma mimicking pulmonary thromboembolism: integrated FDG PET/CT. AJR Am J Roentgenol. 2007; 188:1691–1693.
10. Thurer RL, Thorsen A, Parker JA, Karp DD. FDG imaging of a pulmonary artery sarcoma. Ann Thorac Surg. 2000; 70:1414–1415.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download