Abstract
Duodenal mucinous carcinoma is exceedingly rare and a case report about duodenal mucinous carcinoma in a 61-year-old man mimicking pancreatic cystic neoplasm by radiological evaluation, endoscopy, and even surgical findings is presented.
Although small bowel represents 75% of the length and over 90% of mucosal surface of the alimentary tract, carcinoma of small intestine represents less than one percent of all the malignant tumors of gastrointestinal tract (1). Duodenal adenocarcinoma represents less than 0.3% of all the malignancies in alimentary tract (1) and only three cases have been reported in the medical literature (2, 3, 4). A case report of a duodenal mucinous carcinoma in a 61-year-old man mimicking a pancreatic cystic neoplasm by imaging, endoscopy, and surgery is described.
A 61-year-old man was admitted with complaints of general weakness, myalgia and jaundice for two weeks. The laboratory tests showed an elevated level of aspartate aminotransferase 56 IU/L (normal level: < 37), alanine aminotransferase 124 IU/L (normal level: < 41), total bilirubin 7.0 mg/dL (normal level: < 1.2), alkaline phosphatase 535 IU/L (normal level: < 129), C-reactive protein 10.5 mg/dL (normal level: < 1.2), and leukocyte count 12.2 × 103/mL (normal level: < 4.5-10 × 103) and elevated level of tumor marker of carbohydrate antigen 19-9 104.5 U/mL (normal level: < 37). Physical examination revealed icteric sclera, yellowish skin discoloration and pruritus.
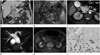
Contrast-enhanced abdominal CT scan showed a smoothly-margined low-attenuating mass in pancreatic head and duodenum. The mass was 3.2 × 3.6 × 4.8 cm, and 25-35 Hounsfield units on both pre- and post-contrast CT images. The tumor caused dilatation of bile duct and main pancreatic duct and showed partly irregular mucosal surface of the duodenum (Fig. 1A).
Endoscopy revealed diffuse ulceration with yellowish exudate on the duodenal mucosa and mucin was secreted from the papilla. Endoscopic ultrasonography showed a heterogeneous hyperechoic mass with scattered anechoic spots and posterior acoustic enhancement (Fig. 1B).
MR imaging of fat suppressed fast imaging employing steady-state acquisition (FIESTA) sequence (repetition time/echo time = 3.72/1.6; flip angle = 70; matrix number = 256 × 224; slice thickness = 5.0 mm) showed a high signal intensity cystic mass in the pancreatic head and duodenum (Fig. 1C). MR cholangiopancreatography showed severe dilatation of the bile duct with segmental luminal narrowing at the distal common bile duct and moderate dilatation of both main and branching pancreatic ducts (Fig. 1D). Fluorine-18 fluorodeoxyglucose positron emission tomography with CT (18F-FDG PET-CT) showed no increased FDG uptake by the mass (Fig. 1E). The various imaging findings suggested an intraductal papillary mucinous neoplasm (IPMN) of pancreas.
The gross specimen of a Whipple procedure showed that the tumor was a soft mass containing large amount of mucin involving the duodenum and the pancreatic head. The microscopic findings showed tumor cell infiltrations with abundant extracellular mucin in the duodenal mucosal and mural portion, direct tumor invasion of the pancreatic head with dilatation of bile and pancreatic ducts and penetration with percolation of interstitial mucin through the duodenal mucosal surface including the papilla. Immunohistochemical staining results were negative in cytokeratin 7 and positive in cytokeratin 19 and 20 (Fig. 1F). Duodenal mucinous carcinoma with invasion of the pancreas was the final pathologic diagnosis.
Mucinous carcinoma is a histologic subtype of adenocarcinoma characterized by abundant mucin production and more than 50% of the tumor component is extracellular mucin (5). Compared with non-mucinous carcinoma, the CT images of mucinous carcinoma of the colon and rectum show a more eccentric bowel wall thickening, heterogeneous with less contrast enhancement, more hypoattenuating areas and a more frequent intratumoral calcification (6). The tumor is hyperintense on T2-weighted MR image and T1-weighted image shows various signal intensity depending on the concentration of mucin (7). The tumor presented also a hypoattenuating eccentric wall thickening mass in the duodenum on contrast-enhanced CT and a high signal intensity cystic tumor on FIESTA MR imaging.
FDG-PET had a low sensitivity for the detection of primary and recurrent mucinous carcinoma of stomach and colorectum (8). Also the cystic mass of pancreatic head and duodenum in the presented patient showed no FDG uptake.
In the presented case, it was not obvious whether the origin of tumor was duodenum or pancreas. The epicenter of the cystic mass in pancreaticoduodenal groove and pancreatic head gave the impression of cystic neoplasm of pancreas on various imaging findings. Even the endoscopic findings and the impression at surgery were that the tumor represented a mucinous neoplasm of the pancreas. The finding of penetration with percolation of interstitial mucin of duodenal mucinous carcinoma was mistaken as mucin secretion of duodenal papilla from IPMN of pancreas. Mucin secretion from duodenal papilla could be seen in IPMN among the various pancreatic cystic neoplasms (9). However, the immunohistochemical staining results and the microscopic findings of irregular margin of tumor infiltration of the pancreatic head and transmural tumor involvement of the duodenum suggested the duodenal origin of the tumor in the presented case. Mucinous carcinomas of colorectum and stomach mostly have an unfavorable prognosis (5, 6, 7, 10). However, neither imaging nor clinical evidence of tumor recurrence has been demonstrated in the patient for more than sixty months.
In conclusion, the duodenal mucinous carcinoma is extremely rare and might mimic a pancreatic cystic neoplasm by imaging, endoscopy and even gross morphologic findings on surgery.
Figures and Tables
Fig. 1
Duodenal mucinous carcinoma with invasion of pancreas in a 61-year-old male patient.
A. Contrast-enhanced CT image with coronal reformation shows a low-attenuating mass with partly irregular mucosal surface (arrowhead) in the duodenum, and dilatation of the pancreatic duct (arrow) and the bile duct (curved arrow).
B. Endoscopic ultrasonography shows heterogeneous hyperechoic mass (arrows) with scattered anechoic spots.
C. Axial fast imaging employing steady-state acquisition MR imaging shows a high signal-intensity cystic mass (arrow) bulging into the duodenal lumen and irregular tumor infiltration (arrowheads) around the distal common bile duct in the pancreatic head.
D. MR cholangiopancreatography shows severe dilatation of the bile duct with segmental luminal narrowing (arrow) at the distal common bile duct and moderate dilatation of the main pancreatic duct (arrowhead) and branches (curved arrow) of pancreatic ducts.
E. Fluorodeoxyglucose positron emission tomography-CT fusion imaging shows no increased uptake in the mass of pancreatic head (arrows).
F. Photomicroscopic pathology (hematoxylin and eosin × 10, cytokeratin immunohistochemistry 7, 19, and 20) shows tumor cell infiltrations in the duodenal mucosa and mural layers with abundant extracellular mucin (arrows) and positive staining in cytokeratin 19 and 20 and negative in 7.
References
1. DiSario JA, Burt RW, Vargas H, McWhorter WP. Small bowel cancer: epidemiological and clinical characteristics from a population-based registry. Am J Gastroenterol. 1994; 89:699–701.
2. Okumura F, Senoo K, Yoshida M, Miyabe K, Naito I, Tanaka H, et al. [A case of peritoneal dissemination from mucinous carcinoma of the duodenum, which was associated with tumor thrombosis in the accessory pancreatic duct and successfully treated by chemotherapy]. Nihon Shokakibyo Gakkai Zasshi. 2009; 106:1736–1743.
3. Tsuro K, Matsumoto M, Moriyasu H, Nakatani Y, Sakurai S, Maekawa Y, et al. A case of duodenal mucinous adenocarcinoma infiltrating the cystic duct diagnosed as transpapillary with transnasal endoscopy. Dig Endosc. 2010; 22:246–247.
4. Yagyu T, Aihara T, Murayama M, Kikuchi S, Nakamura E, Hase K, et al. Mucinous carcinoma of the duodenum associated with hereditary nonpolyposis colorectal cancer: report of a case. Surg Today. 2006; 36:1129–1132.
5. Rosai J. Rosai and Ackerman's Surgical Pathology. In : Rosai J, editor. Gastrointestinal tract: esophagus, stomach, small bowel, appendix, large bowel, anus. 10th ed. Philadelphia: Elsevier-Mosby;2011. p. 731–755.
6. Ko EY, Ha HK, Kim AY, Yoon KH, Yoo CS, Kim HC, et al. CT differentiation of mucinous and nonmucinous colorectal carcinoma. AJR Am J Roentgenol. 2007; 188:785–791.
7. Hussain SM, Outwater EK, Siegelman ES. Mucinous versus nonmucinous rectal carcinomas: differentiation with MR imaging. Radiology. 1999; 213:79–85.
8. Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000; 174:1005–1008.
9. Itoh S, Ishiguchi T, Ishigaki T, Sakuma S, Maruyama K, Senda K. Mucin-producing pancreatic tumor: CT findings and histopathologic correlation. Radiology. 1992; 183:81–86.
10. Kawamura H, Kondo Y, Osawa S, Nisida Y, Okada K, Isizu H, et al. A clinicopathologic study of mucinous adenocarcinoma of the stomach. Gastric Cancer. 2001; 4:83–86.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download