Abstract
Intraluminal duodenal diverticulum (IDD) is a rare congenital anomaly. IDD can become symptomatic in 20% to 25% of cases when complicated by intestinal obstruction, pancreatitis, or hemorrhage. We report the case of a 21-year-old female presenting with IDD mimicking duodenoduodenal intussusception. We describe the imaging features of IDD on the gadoxetic acid-enhanced magnetic resonance image as well as computed tomography.
Intraluminal duodenal diverticulum (IDD) is a rare congenital abnormality consisting of a sac-like mucosal projection in the second portion of the duodenum, in close proximity to the ampulla of Vater. Although the majority of cases are asymptomatic, symptomatic IDD may result in pancreatitis, cholangitis, peptic ulcer disease, gastrointestinal bleeding, and intestinal obstruction (1).
We present a case of IDD mimicking duodenoduodenal intussusception, diagnosed by computed tomography (CT) and gadoxetic acid-enhanced magnetic resonance imaging (MRI) without surgical correction.
A 21-year-old female was transferred to the emergency room with acute postprandial epigastric pain, nausea, and vomiting. A physical examination revealed epigastric tenderness without rebound or guarding. Laboratory data revealed raised levels of serum amylase (806.1 IU/L, normal range 36-128 IU/L) and serum lipase (1689.6 U/L, normal range 22-51 U/L), suggesting a diagnosis of pancreatitis. The patient's leukocyte count was also elevated (15790/uL, normal range 4000-11000/uL). All other blood and urine chemical parameters were within normal limits.
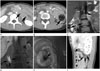
An initial contrast-enhanced CT, obtained at an outside institution, revealed an invagination of the second part of the duodenum, which continued into the fourth part (Fig. 1A). This "bowel-within-bowel" configuration was suggestive of intussusception. Food materials were observed at the end of the intussusceptum, but no tumorous lesions were identified on the CT scan (Fig. 1B). A gadoxetic acid-enhanced MRI was performed to ascertain the cause of the duodenoduodenal intussusception. Following the MRI, intussusception was not detected in the same location compared as in the initial CT scan. Coronal thin-slab T2-weighted fast spin-echo MRI revealed a fluid-filled, saclike structure, surrounded by normal duodenal mucosa, arising just below the ampulla of Vater (Fig. 1C). The coronal gadoxetic acid-enhanced T1-weighted image, obtained 60 minutes following the injection, also revealed a contrast-filled sac in the duodenal lumen (Fig. 1D). Contrast materials were filled in both the sac and duodenal lumen. An endoscopy revealed a sac-like, thickened mucosal fold with another opening immediately distal to the ampulla of Vater (Fig. 1E). Twenty-seven days post-admission, a follow-up CT was performed to monitor the patient's response to treatment for pancreatitis. The sagittal reformatted, contrast-enhanced CT also revealed a fluid-filled sac situated entirely within the duodenum (Fig. 1F). These CT, MRI, and endoscopic findings were consistent with a diagnosis of IDD.
The physicians recommended the patient undergo excision of the intraluminal pouch, but the patient refused surgical intervention. As such, she was conservatively treated with antibiotics to prevent pancreatic necrosis, infection, and organ failure. The patient was discharged on day 27 post-admission, at which time she was asymptomatic, with all laboratory values at a normal level.
IDD is a rare congenital duodenal anomaly that represents a subtype of duodenal web or diaphragm (1). Duodenal web or diaphragm results from a failure of the recanalization process, in the primitive foregut, between weeks 5 and 12 of gestation. A partial or fenestrated duodenal web will gradually transform into intraluminal diverticulum due to chronic anterograde enteric peristalsis (2). The distended diverticulum mimics a large tumor in the lumen, thereby contributing to the production of epigastric pain, postprandial fullness, and vomiting. However, symptoms of IDD are not present when the duodenum is empty, during which time the diverticulum has a tendency to collapse (1).
IDD usually occurs at the second portion of the duodenum and arises near the ampulla of Vater (1). If the IDD is distended with food and repetitive peristalsis extends to the distal duodenum or proximal jejunum, it may mimic intussusception. In some cases, IDD mimicking intussusception has been reported (3, 4). In our case, the initial diagnostic impression was also duodenoduodenal intussusception.
Complications of IDD have been reported in 20-25% of adult patients (1). Reported complications include duodenal obstruction, peptic ulcer disease, gastrointestinal hemorrhage, pancreatitis, and cholangitis (1). Acute pancreatitis was the presenting complication in this patient. Because the patient's ampulla was located immediately proximal to the opening of the diverticulum, partial obstruction of the duodenum by the distended diverticulum caused the reflux of duodenal content through the ampulla of Vater, leading to pancreatitis (5).
Previously, IDD was diagnosed by upper gastrointestinal series. IDD has a pathognomonic radiological appearance, manifesting as a barium-filled sac surrounded by a thin radiolucent halo representing the wall of the diverticulum within the duodenal lumen; the so-called "windsock" sign (6). Endoscopic examination is necessary for the diagnosis in cases with an extremely small opening; endoscopy is also helpful in evaluating its relationship to the ampulla of Vater, and to exclude the presence of a peptic ulcer. In endoscopy, IDD appears as a blind sac with an orifice, and as a polyp when inverted (7).
Currently, IDD diagnoses are made with cross-sectional imaging using CT or MRI. Several reports have described CT findings of IDD, and one report has described MRI findings of IDD (4, 5, 8). CT and MRI reveal both a fluid-filled sac within the duodenum, similar to the pathognomonic "windsock" appearance evidenced in classic barium studies (4, 8). In particular, the T2-weighted MRI is useful for demonstrating fluid accumulation, surrounded by a hypointense rim, in the duodenum (8). The shape and direction of the sac may be altered by peristalsis in each series. Cognizance of this fact is also important for the diagnosis of IDD with CT or MRI. Additionally, CT and MRI are superior to barium studies for evaluating concomitant congenital anomalies and complications associated with IDD (5, 8). Thus, CT and MRI can be used as alternatives to barium studies for the diagnosis of IDD.
However, there are certain cases of IDD which are not identifiable by CT or MRI due to inadequate filling of the IDD by fluid or foods, circumstances under which only thickened, wall-like lesions are visualized (8). In these cases, the hepatobiliary phase image of gadoxetic acid-enhanced MRI may be useful to reveal the attachment of the diverticulum to the duodenal wall. Because 50% of the injected dose of gadoxetic acid is excreted via the biliary tree, contrast material through the ampulla of Vater passes not only through the diverticular sac though the false lumen, but also through the true duodenal lumen. The contrast-filled sac is surrounded by a narrow hypointese line that is easily visible as the contrast in the duodenum passes distal to the diverticulum, which is almost the same as the "windsock" sign seen in the barium study. Moreover, the hepatobiliary phase may yield superior detection of the diverticular sac compared with T2-weighted MRI images, due to the contrast filling.
However, a delay of 30 minutes or more following a gadoxetic acid injection may be necessary in order for contrast materials to fill the sac sufficiently such that it is opacified. Generally, a 20 minute delay after gadoxetic acid administration is sufficient in patients with normal hepatobiliary function (9). In patients with suspected bile leakage or biliary obstruction, biliary opacification is sometimes inadequate on a 20 minute delayed hepatobiliary phase (10). Thus, our protocol for magnetic resonance cholangiopancreatography using gadoxetic acid included an additional 60 minute delayed hepatobiliary phase; a contrast-filled IDD could be identified during the hepatobiliary phase in our case using the identified protocol.
Although surgery (duodenotomy and excision of the diverticulum) represents the treatment of choice, endoscopic incision is strongly advocated, as it is potentially equally efficacious (7).
In conclusion, we report a rare case of IDD as diagnosed by a contrast-enhanced CT and gadoxetic acid-enhanced MRI. Although IDD is very uncommon, it should be borne in mind in the differential diagnosis of duodenoduodenal intussusception. Additionally, this is the first reported case pertaining to IDD, of gadoxetic-enhanced MRI findings. Gadoxetic acid-enhanced MRI, as well as CT, can aid in the diagnosis of IDD, through the contrast-filled diverticulum.
Figures and Tables
Fig. 1
Intraluminal duodenal diverticulum in a 21-year-old female.
A. Axial contrast-enhanced CT at an external institution shows bowel within bowel configuration (arrows) at the 4th portion of the duodenum.
B. Axial contrast-enhanced CT at a more inferior level than A shows food materials (arrow) at the distal end.
C. Coronal T2-weighted thin-slab fast spin-echo MRI shows fluid-filled sac surrounded by a hypointense rim (arrows) in the descending duodenum.
D. Coronal gadoxetic acid-enhanced T1-weighted image obtained 60 min following injection shows a distended intraluminal diverticulum (arrows) compared to C, due to contrast filling.
E. Endoscopy shows intraluminal polypoid swelling in the descending duodenum. A diverticular opening (arrow) is observed at the swelling.
F. Follow-up contrast-enhanced CT with sagittal reformation shows an intraluminal diverticulum with windsock appearance (arrows) in the descending duodenum.
References
1. Afridi SA, Fichtenbaum CJ, Taubin H. Review of duodenal diverticula. Am J Gastroenterol. 1991; 86:935–938.
2. Boyden EA, Cope JG, Bill AH Jr. Anatomy and embryology of congenital intrinsic obstruction of the duodenum. Am J Surg. 1967; 114:190–202.
3. Griffin M, Carey WD, Hermann R, Buonocore E. Recurrent acute pancreatitis and intussusception complicating an intraluminal duodenal diverticulum. Gastroenterology. 1981; 81:345–348.
4. Fidler JL, Saigh JA, Thompson JS, Habbe TG. Demonstration of intraluminal duodenal diverticulum by computed tomography. Abdom Imaging. 1998; 23:38–39.
5. De Rai P, Castoldi L, Tiberio G. Intraluminal duodenal diverticulum causing acute pancreatitis: CT scan diagnosis and review of the literature. Dig Surg. 2000; 17:288–292.
6. Materne R. The duodenal wind sock sign. Radiology. 2001; 218:749–750.
7. Finnie IA, Ghosh P, Garvey C, Poston GJ, Rhodes JM. Intraluminal duodenal diverticulum causing recurrent pancreatitis: treatment by endoscopic incision. Gut. 1994; 35:557–559.
8. Takamatsu S, Gabata T, Matsui O, Noto M, Ninomiya I, Nonomura A. Intraluminal duodenal diverticulum: MR findings. Abdom Imaging. 2006; 31:39–42.
9. Lee NK, Kim S, Lee JW, Lee SH, Kang DH, Kim GH, et al. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009; 29:1707–1724.
10. Cieszanowski A, Stadnik A, Lezak A, Maj E, Zieniewicz K, Rowinska-Berman K, et al. Detection of active bile leak with Gd-EOB-DTPA enhanced MR cholangiography: comparison of 20-25 min delayed and 60-180 min delayed images. Eur J Radiol. 2013; 82:2176–2182.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download