Abstract
Erdheim-Chester disease is a rare non-Langerhans-cell histiocytosis involving multiple organs. On histological evaluation, lipid-laden histiocyte aggregates in Erdheim-Chester disease is detected, but fat tissue in affected organs is not noted grossly on computed tomography. A 40-year-old man presented with bilateral perirenal masses containing fat tissue. He was diagnosed as perirenal involvement of Erdheim-Chester disease. This report describes a case of Erdheim-Chester disease with perirenal involvement that demonstrates unusual features.
Erdheim-Chester disease (ECD) is a rare non-Langerhans-cell histiocytosis involving multiple organs. Most previous reports of this disease have described musculoskeletal or central nervous system involvement. Abdominal involvement has been reported in the perirenal space, ureter, or adrenal gland (1, 2). Here we report a case of ECD with perirenal involvement that demonstrates unusual features with fat tissue on abdominal CT image.
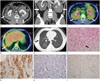
A 40-year-old man presented with dyspnea for one week. He had mild mental retardation and diabetes insipidus. Physical examination and laboratory results were unremarkable. He underwent abdominal CT, chest CT, and positron emission tomography (PET)/CT. The abdominal CT revealed bilateral perirenal masses with bilateral hydronephrosis (Fig. 1A, B). The perirenal masses were first discovered five years earlier on chest CT due to pulmonary tuberculosis. There had been no interval change in the masses since that time. The perirenal masses surrounded both kidneys and even invaded the lower pole of the left kidney. The masses did not compress the kidneys. Fat planes between the kidney and the mass were partially preserved except at the lower pole of the left kidney. Bilateral renal hila were spared. There was no displacement of either kidney or mass effect on adjacent organs. The perirenal masses showed lobulated contours with small amounts of multifocal fat tissue. Intralesional fat tissue demonstrated a comb-like appearance with linear shape perpendicular to the kidney surface. After contrast injection, the masses were poorly enhanced with only subtle enhancement in the periphery. The differential diagnosis for these perirenal masses included retroperitoneal fibrosis, lymphoma, liposarcoma, metastasis, and ECD. However, the perirenal masses contained fat tissue, which restricted the differential diagnosis. Fused PET/CT showed diffuse and moderate fluorodeoxyglucose (FDG) activity (maximum standardized uptake value range, 2.2-3.8) in bilateral perirenal masses (Fig. 1C). In addition, some parts of the lung and the pleura on fused PET/CT showed mild patchy FDG activity (Fig. 1D). On chest CT, multifocal ground glass opacities with interlobular and intralobular septal thickening and multifocal thickening of the right pleura were noted in both lungs, suggestive of interstitial fibrosis (Fig. 1E).
CT-guided gun biopsy with an 18-G core needle was performed to further evaluate the perirenal masses. On histological evaluation with hematoxylin and eosin staining, fibrous tissue proliferation with lipid-laden histiocyte aggregates was detected (Fig. 1F). On immunohistochemical staining, CD68 was positive but CD1a and S-100 (markers of dendritic cells) were negative (Fig. 1G-I). These results were consistent with ECD. Wedge resection of the right upper and lower lung lobes was performed. The lung specimen showed subpleural and interstitial fibrosis with mild chronic inflammation and proliferation of smooth muscle and bronchiolar epithelium, consistent with interstitial pneumonia. On immunohistochemistry of the lung specimen, CD68 was positive.
After glucocorticoids administration for symptom control, dyspnea was relieved. During follow-up, he underwent recurrent urinary tract infection due to vesicoureteral reflux. Foley catheter has indwelled due to neuromuscular dysfunction of urinary bladder. At serial follow-up CT for chest and abdomen, the perirenal masses and abnormal findings of lung and pleura showed no significant change.
ECD was first described by William Chester in 1930 (3). Erdheim-Chester disease is a rare systemic xanthogranulomatous infiltrative disease in which lipid-laden histiocytes deposit in various organs, including long tubular bones, lung, heart, kidney, retroperitoneum, breast, brain, skin, and orbit (4, 5). It usually affects adults over 40 years with a slight male predominance (3, 5, 6). Radiologic examinations in previous reports have described bilateral and symmetric involvement (1, 2, 6, 7). Patients with ECD frequently have diabetes insipidus and central nervous system involvement (8, 9). The cause of ECD is unknown (1). However, its monoclonal proliferation of histiocytes suggests neoplastic nature (9). Diagnosis of ECD can be established by histological findings of tissue infiltration by foamy histiocytes without cytoplasmic Birbeck granules and positive immunohistochemical staining for CD68 but negative staining for CD1a and S-100 (1). The clinical course of ECD is variable depending on the involved organ and the extent of the disease (3). Treatments for ECD include steroids, chemotherapy, radiation therapy, immunotherapy, and surgery (9).
In this case, our patient with ECD had diabetes insipidus, lung involvement with interstitial lung disease, and perirenal involvement. Long tubular bone involvement occurs frequently in patients with ECD. However, there was no bone involvement in this case. Patients with ECD often have perirenal involvement. Previously, one report described this perirenal involvement as 'hairy kidneys' (1). A review of diseases in the perirenal space classified ECD as one of the rind-like soft-tissue lesions based on the distribution and imaging features (6). In this case, the patient had perirenal involvement with imaging features slightly different from previous reports. First, the perirenal masses contained macroscopic fat tissue. To the best of our knowledge, there have been no other case of ECD with this finding. Histologically, sheets and cords of foamy histiocytes diffusely infiltrate the tissues and viscera with ECD (3). In ECD, perirenal mass formed by lipid-laden macrophages' aggregation proven on histologic examination failed to show fat attenuation at CT. The macroscopic fat tissue within the bilateral perirenal masses may be preexisting perirenal fat engulfed by aggregation of foamy histiocytes rather than aggregated lipid-laden macrophage itself. Second, perirenal masses surrounded both kidneys. However, there was no severe compression of renal parenchyma or ureters. In addition, the fat plane between kidney and perirenal mass was partially preserved. Pelvocalyceal dilatation was thought to be caused by neurogenic bladder and vesicoureteral reflux due to the patient's physical disability inflicted by idiopathic brain atrophic changes rather than ureteral obstruction causing the pelvocalyceal dilatation. In previous reports of ECD, no space was found between the kidneys and the rind-like soft tissue lesion surrounding them (6). Third, perirenal masses in this case had a lobulated contour with clear margins without perirenal fat infiltration. Typically, fat infiltration in the perirenal space coexists with ECD.
In conclusion, we described a patient with Erdheim-Chester disease with perirenal masses containing macroscopic fat tissue. Perirenal involvement of ECD may present as bilateral perirenal masses with macroscopic fat tissue.
Figures and Tables
Fig. 1
A 40-year-old man with Erdheim-Chester disease. Bilateral perirenal masses with faint fat tissue (white arrows) are revealed on axial images (A) and coronal image of abdominal CT (B). Fused positron emission tomography (PET)/CT shows diffuse and moderate fluorodeoxyglucose (FDG) activity (maximum standardized uptake value range, 2.2-3.8) in bilateral perirenal masses (white arrows) (C). Some parts of lung and pleura (white arrow) also show mild patchy FDG activity on fused PET/CT (D). Multifocal ground glass opacities with interlobular and intralobular septal thickening and thickening of the right pleura (black arrow) are noted on chest CT (E). H&E stain (× 400) indicates lipid-laden histiocyte aggregation (black arrow for one among many lipid-laden histiocyte) (F). Immunohistochemical stains show that CD68 (× 200) (G) is positive and CD1a (× 200) (H) and S-100 (× 200) (I) are negative.
References
1. Alberti N, Frulio N, Bertolotti A, Petitpierre F, Veron A, Perez JT, et al. Erdheim-Chester disease: a rare diagnosis with evocative imaging. Diagn Interv Imaging. 2013; 94:457–459.
2. Heller MT, Haarer KA, Thomas E, Thaete FL. Neoplastic and proliferative disorders of the perinephric space. Clin Radiol. 2012; 67:e31–e41.
3. Dickson BC, Pethe V, Chung CT, Howarth DJ, Bilbao JM, Fornasier VL, et al. Systemic Erdheim-Chester disease. Virchows Arch. 2008; 452:221–227.
4. De Filippo M, Ingegnoli A, Carloni A, Verardo E, Sverzellati N, Onniboni M, et al. Erdheim-Chester disease: clinical and radiological findings. Radiol Med. 2009; 114:1319–1329.
5. Lee HJ, Lee KY, Shin DY, Lee YG, Choi SY, Moon KC, et al. A case of erdheim-chester disease with asymptomatic renal involvement. Cancer Res Treat. 2012; 44:146–150.
6. Surabhi VR, Menias C, Prasad SR, Patel AH, Nagar A, Dalrymple NC. Neoplastic and non-neoplastic proliferative disorders of the perirenal space: cross-sectional imaging findings. Radiographics. 2008; 28:1005–1017.
7. Provenzano E, Barter SJ, Wright PA, Forouhi P, Allibone R, Ellis IO. Erdheim-chester disease presenting as bilateral clinically malignant breast masses. Am J Surg Pathol. 2010; 34:584–588.
8. Adam Z, Balsíková K, Krejcí M, Pour L, Stěpánková S, Svacina P, et al. [Central diabetes insipidus in adult patients--the first sign of Langerhans cell histiocytosis and Erdheim-Chester disease. Three case studies and literature review]. Vnitr Lek. 2010; 56:138–148.
9. Adem C, Hélie O, Lévêque C, Taillia H, Cordoliani YS. Case 78: Erdheim-Chester disease with central nervous system involvement. Radiology. 2005; 234:111–115.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download