Abstract
Purpose
To evaluate the multiplicity and mean number of cystic lesions in patients with branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), serous cystadenomas (SCAs), and mucinous cystic neoplasms (MCNs) of the pancreas.
Materials and Methods
Two hundred and eighty-eight patients with pathologically proven cystic neoplasms of the pancreas underwent preoperative CT and/or MRI. These patients were divided into the following three groups: BD-IPMN, SCA, and MCN groups. Two radiologists retrospectively analyzed the CT and MRI examinations to determine the multiplicity (i.e., more than one lesion) and mean number of cystic lesions per patient. Among the three groups, statistical comparison of multiplicity of cystic lesions was performed.
Results
The BD-IPMN group consisted of 155 patients with 176 BD-IPMNs, with multiplicity in 15 patients (9.7%) and a mean 1.14 BD-IPMNs per patient (range, 1.3). The SCA group consisted of 67 patients with 69 SCAs, with multiplicity in one patient (1.5%) and a mean 1.03 SCAs per patient (range, 1.3). The MCN group consisted of 67 patients with 67 MCNs, with no multiplicity. Multiple cystic lesions were significantly more common in the BD-IPMN group than in the SCA and MCN groups (p = 0.003).
Cystic lesions of the pancreas are increasingly being detected due to the more widespread use of and advances in cross-sectional imaging (CT and MRI) and the greater frequency of health examinations. Cystic lesions of the pancreas may be congenital or developmental; neoplastic or non-neoplastic; epithelial or mesenchymal; and true or degenerative. Serous cystadenomas (SCAs), mucinous cystic neoplasms (MCNs), and intraductal papillary mucinous neoplasms (IPMNs) account for 90% of all primary pancreatic cystic neoplasms (1). SCAs are considered to be benign, and in the absence of symptoms, they can be safely monitored radiographically (2). However, MCNs and IPMNs should be resected due to their varying degrees of malignant potential (2, 3, 4, 5, 6, 7). The likelihood of malignancy is significantly higher in patients with main duct and combined IPMNs than in those with branch duct IPMNs (BD-IPMNs) (4, 6, 7). The management of patients with IPMNs is unclear (5, 8, 9). BD-IPMNs less than 3 cm in diameter without main pancreatic duct (MPD) dilatation or mural nodules can be managed conservatively without treatment (8, 9, 10). Therefore, accurate preoperative characterization of cystic neoplasms of the pancreas is important due to the different patterns of prognosis and differences in patient management.
CT and MRI are modalities used in both the detection and characterization of cystic pancreatic neoplasms. The characteristic features detected by CT and MRI are locularity (unilocular, oligolocular, or multi-locular), internal cysts (microcystic or macrocystic), MPD communication, presence of mural nodules, and central or peripheral calcifications (6, 11, 12). Endoscopic retrograde cholangiopancreatography (ERCP) is the imaging modality of choice for the diagnosis of IPMN due to the detection of communication between the dilated branch ducts and the MPD or the detection of intraductal mucin.
However, more recent studies have found that CT and MRI have diagnostic accuracies ranging from 27% to 96.8% in detecting pancreatic cystic neoplasms, which were lower than expected (13, 14, 15). Although imaging findings and differentiation of pancreatic cystic neoplasms have been reported in many studies, their accurate radiologic differentiation is still difficult due to their overlapping morphologies and their very small sizes for evaluation (12, 13, 14, 15, 16, 17, 18, 19, 20, 21).
In daily clinical practice, we observed that BD-IPMNs were more frequently associated with multiple cystic lesions than MCNs and SCAs. We hypothesized that there would be significant differences in the multiplicity of cystic lesions in patients with BD-IPMN, SCA, and MCN of the pancreas, and that these differences may be helpful in differentiating among various types of pancreatic cystic neoplasms. However, to the best of our knowledge, the multiplicity and mean number of cystic lesions per patient have not been evaluated. Therefore, we evaluated the multiplicity and mean number of cystic lesions in patients with BD-IPMN, SCA, and MCN of the pancreas.
Our Institutional Review Board approved this retrospective study and waived the requirement for informed consent. A computer search of the pathology database at our institution for patients with BD-IPMN from January 2001 to December 2006, and for patients with SCA and MCN of the pancreas from January 1997 to December 2006 identified 301 patients. Of the patients with IPMN, we selected only those with BD-IPMN because the main duct and combined IPMNs showed different imaging findings of diffuse or segmental dilatation of the MPD compared to those observed in SCAs or MCNs. Patients were included if they had pathologically proven BD-IPMN, SCA, or MCN and had undergone preoperative imaging (i.e., CT, MRI, or ERCP). Thirteen patients were excluded: eight patients had discrepancies in the number of cystic lesions between imaging and pathology results; in three patients with two cystic lesions on imaging, only one cystic lesion, a pathologically proven BD-IPMN, was resected without preoperative ERCP; one patient with both pancreatic ductal adenocarcinoma and BD-IPMN had no cystic lesions on imaging; and one patient with von Hippel-Lindau syndrome had numerous cystic lesions.
The final study group consisted of 288 patients (124 men and 164 women; mean age, 56.5 years), and they were categorized into three groups according to their pathology results (i.e., BD-IPMN, SCA, and MCN groups). The BD-IPMN group consisted of 155 patients (92 men, 63 women; mean age, 61.9 years) with 176 BD-IPMNs; the SCA group consisted of 67 patients (22 men, 45 women; mean age, 49.6 years) with 69 SCAs; and the MCN group consisted of 67 patients (10 men, 57 women; mean age, 50.7 years) with 67 MCNs. One 52-year-old female patient had both BD-IPMN and SCA and was included in both groups, but she was excluded from the analysis of multiplicity of cystic lesions in both groups. In five patients with BD-IPMNs, all cystic lesions were not resected. However, ERCP showed that the non-resected cystic lesions (n = 6) communicated with the MPD, with the resected cystic lesions being classified as BD-IPMNs histopathologically. Therefore, these six non-resected cystic lesions were also considered as BD-IPMNs.
Because this study was retrospective in nature and included CT and MRI performed over a 10-year period, the scanning protocols for the examinations varied. CT scanning was performed in 69 patients with a single-detector row helical CT scanner (HiSpeed Advantage; GE Medical Systems, Milwaukee, WI, USA) and in 216 patients with a multidetector row CT scanner (LightSpeed QX/i; GE Medical Systems, or Somatom Sensation 16; Siemens Medical Solutions, Forchheim, Germany). Triphasic CT [unenhanced, arterial phase (ATP) and portal venous phase (PVP)] was performed in 209 patients and single-phase CT (PVP) was performed in 76 patients. CT scans were obtained during the ATP (using a bolus tracking technique or a fixed 25-sec delay) and the PVP (using a 72-sec delay) after intravenous injection of 150 mL of iopromide (Ultravist 370; Schering, Berlin, Germany) with a power injector (LF CT 9000; Liebel-Flarsheim, Cincinnati, OH, USA) at a rate of 3 mL/sec through an 18-gauge angiographic catheter inserted into an antecubital vein. Single-detector row helical CT images were obtained with 5-7 mm collimation, 1.4 pitch, 5-7 mm reconstruction intervals, 120 kVp, and 200 mA, and multidetector row CT images were obtained with 3-5 mm section thickness, 1-1.5:1 beam pitch, 3-5 mm reconstruction intervals, 0.5-0.6 s gantry rotation time, 120 kVp, and 200-250 mA.
MRI and MR cholangiopancreatography (MRCP) were performed using a 1.5-T MR imaging system (Magnetom Vision or Avanto; Siemens Medical Solutions, Erlangen, Germany) with a phased-array body coil of four or six elements. Before MRCP, T1-weighted axial gradient-echo MR imaging [repetition time (TR)/echo time (TE), 149/4.1; flip angle, 80°; section thickness, 6-8 mm; 18-20 sections; intersection gap, 1.6 mm] and half-Fourier acquisition single-shot turbo spin-echo (HASTE) T2-weighted axial MR imaging (TR/TE, infinite/134; flip angle, 150°; section thickness, 8 mm; 18-20 sections; intersection gap, 1.6 mm) were performed during a single breath-hold. Two different MRCP sequences, single-shot rapid acquisition with relaxation enhancement (RARE) and multislice HASTE, were applied. Thick-slab single-shot RARE images were obtained at various angles to allow optimal visualization of the biliary and pancreatic ducts; the number of thick-slab acquisitions per patient ranged from 5 to 10 (mean, 6 acquisitions). Multislice thin-section HASTE images were subsequently obtained in the coronal plane. The parameters for single-shot RARE imaging were as follows: TR/TE, infinite/1080; echo train length, 256; flip angle, 150°; slab thickness, 50-70 mm; field of view, 300-320 mm; matrix, 512 × 240; and acquisition time, 3.32 sec. The parameters for multislice HASTE imaging were as follows: TR/TE, infinite/144; echo train length, 128; flip angle, 150°; section thickness, 4 mm with no gap; number of sections, 15-19 (volume of coverage, 60-76 mm); field of view, 300-320 mm; matrix, 512 × 128; and acquisition time, 18-23 sec.
ERCP was performed by three experienced gastroenterologists, each with 10-15 years of experience, while patients were consciously sedated. Under fluoroscopic guidance, 10-30 mL of water-soluble contrast material was injected into the pancreatic and biliary ducts, and multiple images of the pancreatic and biliary ducts were obtained with the patient in the prone position to optimally visualize the entire ductal anatomy and to identify any abnormality.
CT (n = 285; BD-IPMN, n = 153; SCA, n = 66; MCN, n = 66) and MR with MRCP (n = 174; BD-IPMN, n = 128; SCA, n = 24; MCN, n = 21; both BD-IPMN and SCA, n = 1) images were retrospectively analyzed by two board-certified abdominal radiologists (with 9 and 3 years of experience in abdominal radiology, respectively) who were blinded to the diagnosis of the pancreatic lesion in consensus. CT and MR images were analyzed at the same time, but ERCP images were analyzed at a separate session. We analyzed the location (the head, including the portion on the right side of the neck of the pancreas; the neck, including the anterior portion of the confluence of the splenic and superior mesenteric veins; the body, including the area between the neck and tail of the pancreas; and the tail, including the portion on the left side of the left lateral border of the spine body), maximum diameter, and number of pancreatic cystic lesions. We also assessed lesion multiplicity (i.e., more than one lesion) and mean number of cystic lesions per patient.
ERCP images and reports were reviewed by one reader, who reviewed CT and MRI images, to identify the communication of the MPD with the pancreatic cystic lesions, in patients with pancreatic cystic lesions which were not histopathologically proven. All images were reviewed by using a local PACS monitor and digital imaging and communications in medicine image viewing software.
One radiologist reviewed the pathology reports of the 288 patients. The reviewer analyzed the location (head, neck, body, or tail), maximum diameter, and number of pancreatic cystic lesions; histologic spectrum of neoplasms (adenoma, borderline, and malignancy); and morphological subtypes of SCAs, as well as the multiplicity and mean number of cystic lesions per patient.
The multiplicity of cystic lesions was compared among the three patient groups (i.e., the BD-IPMN, SCA, and MCN groups) using the Fisher's exact test. Also the patient's age and maximum diameter of cystic lesions were compared among the three groups using the one-way analysis of variance. A post-hoc analysis using the Bonferroni method was performed if the results were significant. A pair-wise comparison (BD-IPMN vs. SCA, SCA vs. MCN, and BD-IPMN vs. MCN groups) using the Fisher's exact test was performed for the comparison of sex of the patients and location of cystic lesions. All statistical analyses were performed using SPSS for Windows (version 21; SPSS Inc., Chicago, IL, USA), with a p-value of less than 0.05 being considered statistically significant.
The BD-IPMN group consisted of 155 patients with 176 BD-IPMNs, including 170 BD-IPMNs that were diagnosed by histopathology (Fig. 1) and six non-resected lesions diagnosed by ERCP (Fig. 2). Of the 155 patients, 15 (9.7%) had multiple BD-IPMNs. The mean number of these cystic lesions per patient was 1.14 (range, 1-3). Of the 176 BD-IPMNs, 108 BD-IPMNs were located in the pancreatic head, 37 BD-IPMNs were located in the tail, 26 BD-IPMNs were located in the body, and 5 BD-IPMNs were located in the neck. The mean maximum lesion diameter was 32.0 mm (range, 6-170 mm). Histologically, 86 BD-IPMNs were adenomas, 57 BD-IPMNs were borderline, and 27 BD-IPMNs were carcinomas.
The SCA group consisted of 67 patients with 69 SCAs, all of which were diagnosed by histopathology. One patient had three SCAs (1.5%, 1/67) (Fig. 3). The mean number of cystic lesions per patient was 1.03 (range, 1-3). Of the 69 SCAs, 33 SCAs were located in the tail, 23 SCAs were located in the head, 10 SCAs were located in the body, and 3 SCAs were located in the neck of the pancreas. The mean maximum lesion diameter was 43.8 mm (range, 3-110 mm). Morphologically, 58 lesions were microcystic SCAs, and 11 lesions were oligocystic or macrocystic SCAs.
The MCN group consisted of 67 patients with 67 histopathologically proven MCNs (Fig. 4). None of the patients had multiple MCNs. Of these 67 MCNs, 46 MCNs were located in the tail, 11 MCNs were located in the head, 9 MCNs were located in the body, and 1 MCN was located in the neck of the pancreas. The mean maximum lesion diameter was 48.7 mm (range, 10-180 mm). Histologically, 63 MCNs were adenomas and 4 were adenocarcinomas.
Table 1 summarizes the multiplicity and mean number of pancreatic cystic lesions in the BD-IPMN, SCA, and MCN groups. Of the 288 patients, one patient had both SCA and BD-IPMN and was therefore included in both the BD-IPMN and SCA groups, but excluded from the analysis of multiplicity of the cystic lesions in each group. The multiplicity of BD-IPMNs was significantly greater than that of SCAs or MCNs (p = 0.003).
The patients in the BD-IPMN group were significantly older than those in the SCA and MCN groups (p < 0.001). The maximum diameter of BD-IPMNs was significantly smaller than that of SCAs and MCNs (p < 0.001). The number of male patients was greater in the BD-IPMN group than in the SCA or MCN groups (p < 0.001). The number of female patients was greater in the MCN group than in the SCA group (p = 0.025). The BD-IPMNs were significantly frequently located in the pancreatic head than the SCAs or MCNs (p < 0.001). The location of cystic lesions was not significantly different between the SCA vs. MCN groups (p = 0.051).
Many studies have reported the imaging findings which can differentiate cystic neoplasms of the pancreas because of the overlapping morphologies of cystic neoplasms of the pancreas and difficulties in their differentiation (12, 13, 15, 19, 20, 21). Using CT cutoffs for both the number (i.e., six) and size (i.e., 2 cm diameter in greatest dimension) of cysts within tumors resulted in the correct diagnosis of SCAs and MCNs, with sensitivities of 93% and 95%, respectively (19). CT findings of lobulated contours, absence of wall enhancement, and location in the pancreatic head were observed more often in unilocular macrocystic SCAs than in MCNs and pseudocysts (21). A simple imaging-based classification system for pancreatic cystic lesions according to the morphologic features may be used to categorize cysts into four subtypes: unilocular cysts, microcystic lesions, macrocystic lesions, and cysts with a solid component (12). More recently, Kim et al. (20) reported that serous oligocystic adenomas of the pancreas showed typical CT findings, such as multicystic or lobulated cystic lesions with septation, that differ from those in other macrocystic neoplasms such as MCNs and BD-IPMNs. However, in daily clinical practice, accurate differential diagnosis of these pancreatic cystic neoplasms is often difficult, because they have overlapping imaging features and are small in size. The communication between dilated branch ducts and MPD on ERCP and/or MRCP images is highly suggestive of BD-IPMNs (4, 22). However, MCNs or microcystic SCAs may also communicate with the MPD with a very lower frequency than BD-IPMNs (18, 23, 24, 25). Therefore, the presence of ductal communication is helpful but not always reliable in the differentiation of pancreatic cystic lesions (16). Furthermore, all BD-IPMNs do not show communication between MPD and dilated branch ducts on ERCP and MRCP.
BD-IPMNs occur predominantly in the head of the pancreas and are most frequently localized. However, 20-30% of BD-IPMNs are multifocal and 5-10% of BD-IPMNs diffusely involve the entire pancreatic gland (3, 26). Multiple BD-IPMNs have been reported in 20-64% of patients with these lesions (3, 9, 27, 28); in 7 (46.7%) of 15 patients after resection (27); in 57 (38.8%) of 147 patients in surgical specimens (n = 29) or follow-up images (n = 28) (28); and in 57 (64%) of 89 patients on follow-up images (9). Our patients, however, had a lower incidence of BD-IPMN multiplicity (9.7%), which may be due to differences in study design and patient sample size. For example, we did not include BD-IPMNs diagnosed after the follow-up imaging and without pathologic confirmation. If we had included these BD-IPMNs which were diagnosed on imaging and follow-up imaging, the multiplicity of BD-IPMNs would have been higher.
To the best of our knowledge, there have been no studies assessing the multiplicity of SCAs or MCNs in the English language literature. We therefore evaluated the multiplicity and mean number of cystic lesions in the BD-IPMN, SCA, and MCN groups to determine if these factors allow more accurate diagnosis and differentiation. We observed significant differences in multiplicity of cystic lesions among these three groups (p = 0.003). Patients with BD-IPMN had a significantly higher rate of multiplicity than patients with SCA or MCN, suggesting that the multiplicity of pancreatic cystic neoplasms may be helpful in differentiating among patients with BD-IPMN, SCA, and MCN.
As demonstrated in our study, the patients with BD-IPMNs were older and more commonly males than those in the SCA or MCN groups. Furthermore, BD-IPMNs occurred frequently in the head of the pancreas. In addition to multiplicity, these results may also help to differentiate BD-IPMN from SCA or MCN. BD-IPMNs were significantly smaller than SCAs or MCNs in our study. However, the value of size criteria for this differentiation is doubtful because the sizes of cystic lesions of BD-IPMN, SCA, and MCN are variable, especially according to the SCA subtype.
Our study had several limitations. First, we included six non-resected BD-IPMNs communicating with the MPD on ERCP images and co-occurring with pathologically confirmed BD-IPMNs. As mentioned above, MCNs and microcystic SCAs can communicate with the MPD (18, 23, 25, 26). Therefore, these six BD-IPMNs may actually have been other types of cystic tumors, although this was highly unlikely. However, even after excluding these BD-IPMNs, the rate of multiplicity remained significantly greater for BD-IPMNs than for SCAs and MCNs (6.5%, 10/155, p = 0.037). Another limitation of this study was the inclusion of only those patients with pathologically confirmed BD-IPMN, SCA, and MCN except for the six BD-IPMNs mentioned above. In daily practice, small cystic lesions of the pancreas without a solid portion, including multiple small BD-IPMNs, are considered to be benign, and are usually managed with follow-up imaging without surgery. By including only the patients with pathologically confirmed diagnoses, we may have underestimated the real multiplicity of pancreatic cystic neoplasms. A third limitation was the exclusion of patients with rare cystic lesions such as lymphoepithelial cysts. Because these three types (IPMNs, SCAs, and MCNs) account for more than 90% of all primary pancreatic cystic neoplasms (1), other rare cystic neoplasms may not have influenced the results of this study. Fourth, because this study was a retrospective analysis of patients evaluated over a 10-year period, the CT and MRI protocols varied. Therefore, we may have missed lesions on earlier images, which may have affected the lesion multiplicity. Finally, we only analyzed the multiplicity of cystic lesions for their differential diagnosis. However, the morphologic features of cystic lesions are important for radiologic diagnosis. Therefore, further studies regarding both the multiplicity and morphologic features of cystic lesions are needed to differentiate among the different types of pancreatic cystic neoplasms. Despite these limitations, this study, which included a relatively large number of patients, is the first to evaluate the multiplicity and mean number of pathologically proven BD-IPMNs, SCAs, and MCNs of the pancreas to enhance the accuracy of their diagnosis.
In conclusion, the rate of multiplicity was significantly higher for BD-IPMNs than for SCAs or MCNs of the pancreas. Multiplicity of pancreatic cystic neoplasms may be helpful in differentiating among different types of pancreatic cystic neoplasms.
Figures and Tables
Fig. 1
A 71-year-old man with two branch duct IPMNs (diameter, 29 mm and 15 mm) in the body and neck of the pancreas.
A, B. Contrast-enhanced CT images show two lobulated cystic lesions (arrows) in the body and neck of the pancreas.
C. MRCP image shows two multi-lobulated cystic lesions (arrows) in the body and neck of the pancreas.
D. Photograph of gross specimen shows two branch duct IPMNs. Histologically, the IPMNs were adenomas.
Note.-IPMN = intraductal papillary mucinous neoplasm, MRCP = MR cholangiopancreatography
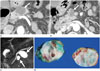
Fig. 2
A 45-year-old man with two branch duct IPMNs (diameter, 19 mm and 22 mm) in the head and tail of the pancreas.
A, B. Contrast-enhanced CT images show two lobulated cystic lesions (arrows) in the head and tail of the pancreas.
C. MRCP image shows two multi-lobulated cystic lesions (arrows) in the head and tail of the pancreas.
D. ERCP image shows two contrast-filling outpouching lesions (arrows), indicating ductal communication.
E. Photograph of gross specimen shows a pathologically proven branch duct IPMN (arrow) in the pancreatic head. The other branch duct IPMN in the pancreas tail was diagnosed by ERCP.
Note.-ERCP = endoscopic retrograde cholangiopancreatography, IPMN = intraductal papillary mucinous neoplasm, MRCP = MR cholangiopancreatography
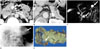
Fig. 3
A 32-year-old woman with three SCAs (diameter, 11 mm, 25 mm, and 45 mm, respectively) in the tail of the pancreas.
A, B. Contrast-enhanced CT images show two unilocular cystic lesions (white arrows) and a multilocular cystic lesion (black arrows) in the tail of the pancreas.
C. Photograph of gross specimen shows pathologically proven two SCAs (arrows) in the pancreatic tail. The smallest lesion was not seen in this photograph of gross specimen. Histologically, the SCAs were oligocystic (marcocystic) adenomas.
Note.-SCA = serous cystadenoma

Fig. 4
A 40-year-old woman with MCN (diameter, 32 mm) in the tail of the pancreas.
A. Contrast-enhanced CT image shows a septated cystic lesion (arrow) in the tail of the pancreas.
B. Axial T2-weighted image shows a multilocular cystic lesion (arrow) in the tail of the pancreas.
C. Photograph of gross specimen shows pathologically proven MCN (arrow) in the pancreatic tail. Histologically, the MCN was adenoma.
Note.-MCN = mucinous cystic neoplasm

References
1. Fernández-del Castillo C, Warshaw AL. Cystic tumors of the pancreas. Surg Clin North Am. 1995; 75:1001–1016.
2. Federle MP, McGrath KM. Cystic neoplasms of the pancreas. Gastroenterol Clin North Am. 2007; 36:365–376. ix
3. Campbell F, Azadeh B. Cystic neoplasms of the exocrine pancreas. Histopathology. 2008; 52:539–551.
4. Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas. 2004; 28:282–288.
5. Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002; 123:1500–1507.
6. Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004; 239:678–685. discussion 685-687.
7. Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003; 90:1244–1249.
8. Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006; 6:17–32.
9. Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007; 56:1086–1090.
10. Irie H, Yoshimitsu K, Aibe H, Tajima T, Nishie A, Nakayama T, et al. Natural history of pancreatic intraductal papillary mucinous tumor of branch duct type: follow-up study by magnetic resonance cholangiopancreatography. J Comput Assist Tomogr. 2004; 28:117–122.
11. Hutchins GF, Draganov PV. Cystic neoplasms of the pancreas: a diagnostic challenge. World J Gastroenterol. 2009; 15:48–54.
12. Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005; 25:1471–1484.
13. Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000; 175:99–103.
14. Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999; 23:906–912.
15. Song SJ, Lee JM, Kim YJ, Kim SH, Lee JY, Han JK, et al. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: comparison of multirow-detector CT and MR imaging using ROC analysis. J Magn Reson Imaging. 2007; 26:86–93.
16. Choi EK, Park SH, Kim DY, Kim KW, Byun JH, Lee MG, et al. Unusual manifestations of primary pancreatic neoplasia: Radiologic-pathologic correlation. J Comput Assist Tomogr. 2006; 30:610–617.
17. Procacci C, Graziani R, Bicego E, Bergamo-Andreis IA, Guarise A, Valdo M, et al. Serous cystadenoma of the pancreas: report of 30 cases with emphasis on the imaging findings. J Comput Assist Tomogr. 1997; 21:373–382.
18. Procacci C, Graziani R, Bicego E, Bergamo-Andreis IA, Mainardi P, Zamboni G, et al. Intraductal mucin-producing tumors of the pancreas: imaging findings. Radiology. 1996; 198:249–257.
19. Johnson CD, Stephens DH, Charboneau JW, Carpenter HA, Welch TJ. Cystic pancreatic tumors: CT and sonographic assessment. AJR Am J Roentgenol. 1988; 151:1133–1138.
20. Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, et al. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006; 187:1192–1198.
21. Cohen-Scali F, Vilgrain V, Brancatelli G, Hammel P, Vullierme MP, Sauvanet A, et al. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology. 2003; 228:727–733.
22. Sugiyama M, Atomi Y, Kuroda A. Two types of mucin-producing cystic tumors of the pancreas: diagnosis and treatment. Surgery. 1997; 122:617–625.
23. Procacci C, Megibow AJ, Carbognin G, Guarise A, Spoto E, Biasiutti C, et al. Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics. 1999; 19:1447–1463.
24. Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999; 230:152–161.
25. Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, Kimura W, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004; 28:241–246.
26. Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol. 2007; 20:Suppl 1. S71–S93.
27. Lim JH, Yoon KH, Kim SH, Kim HY, Lim HK, Song SY, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004; 24:53–66. discussion 66-67.
28. Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007; 102:1759–1764.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download