Abstract
Extrarenal manifestations of autosomal dominant polycystic kidney disease (ADPKD) include non-renal, various intracranial, and cardiac cysts. A 26-year-old man with presumed ADPKD was also diagnosed with hemoptysis and dyspnea. The chest radiograph and CT scans indicated bilateral consolidations, ground-glass opacities, and bilateral pleural effusions, indicating symptoms of pulmonary edema. Based on the systolic dysfunction, left atrial enlargement, and left ventricular enlargement by echocardiography, he was diagnosed with dilated cardiomyopathy (DCMP). On screening brain CT angiography, an intracranial aneurysm (ICA) was detected in the right middle cerebral artery. Although the reason of a strong connection between ADPKD and DCMP is still unclear apart from the connection between ADPKD and ICA, DCMP may be considered as another cardiovascular manifestation of ADPKD when idiopathic DCMP is diagnosed in a patient with ADPKD.
Autosomal dominant polycystic kidney disease (ADPKD) is one of the most commonly inherited renal diseases, and it primarily manifests as multiple, bilateral renal cysts, leading to massive enlargement of the kidneys and progressive renal failure (1). The localization of polycystins, encoded by PKD genes, outside of renal tubules results in various extrarenal manifestations of ADPKD (2). Extrarenal manifestations of ADPKD include non-renal cysts in liver, seminal vesicle, pancreas, and various cardiac manifestations such as valvular abnormalities, left ventricular hypertrophy (LVH), early onset hypertension (HTN), and pericardial effusions. Intracranial manifestations include intracranial aneurysms (ICA) and arachnoid cyst. Other less common extrarenal manifestations are diverticular disease, abdominal hernias, and bronchiectasis (2,3,4). However, to the best of our knowledge, no case report of ADPKD accompanied with ICA and dilated cardiomyopathy (DCMP) is reported. Herein, we report the first case of ADPKD combined with ICA and DCMP diagnosed in a 26-year-old male patient.
A 26-year-old man was admitted to the emergency room for one day; he suffered from presumably ADPKD and also diagnosed with hemoptysis and dyspnea. One month prior to this case, he was diagnosed with asthma and received medical treatment and periodic follow-up in our hospital. His blood pressure had been high for several years; however, he did not receive any anti-hypertensive treatment. His father had undergone kidney transplantation because of end-stage renal disease caused by ADPKD and had a history of ruptured ICA.
Wheezing was heard on auscultation; however, no cardiac murmur was observed. His initial blood pressure and body temperature were 140/80 mm Hg and 36.6℃, respectively. The initial blood test results indicated increased levels of Hb and Hct to 17.2 g/dL and 50.2%, respectively. The white blood cell count and erythrocyte sedimentation rate were 8250 and 10 mm/h, respectively, and were within the normal range. Brain natriuretic peptide was high (441.38 pg/mL), whereas serum creatinine was normal (0.98 mg/dL). The arterial blood gas analysis showed pH, pO2, pCO2, SaO2, and HCO3 of 7.44, 58 mm Hg, 30 mm Hg, 91.2%, and 22.3 mmol/L, respectively, indicating compensated respiratory alkalosis. The urine analysis revealed proteinuria (2+). An electrocardiogram showed left anterior fascicular block, LVH, and sinus tachycardia (112/min). Subsequent echocardiography showed left atrial enlargement (LAE), left ventricular enlargement (LVE), global hypokinesia of the LV wall with severe systolic dysfunction [ejection fraction (EF), 21%], and an increased LV mass index (189 g/m2) (Fig. 1A). However, no evidence of cardiac valve abnormality or coronary artery aneurysm was observed.
Initial chest radiograph and CT scans, performed to rule out pulmonary causes of hemoptysis, revealed bilateral consolidations, ground-glass opacities (GGO), cardiomegaly, and bilateral pleural effusions, indicating pulmonary edema (Fig. 1B). The diameter of main pulmonary artery trunk was 3.4 cm, indicating pulmonary HTN. Based on the systolic dysfunction, LAE, and LVE, the patient was suspected of having DCMP. On abdominal CT scan, both kidneys showed enlargement and innumerable variable-sized cysts, indicative of ADPKD (Fig. 1C). Two small simple cysts were also seen in the liver. Brain CT angiography was performed to exclude the possibility of ICA because of his family history of ruptured ICA, and a 4 mm saccular aneurysm was detected in the right middle cerebral artery (MCA) (Fig. 1D). Endovascular coiling for preventive treatment of the right MCA aneurysm was considered but postponed because of the patient's cardiopulmonary problems. Digoxin (Digosin Tab, CJ HealthCare, Seoul, Korea, 5 mg), Losartan (Cozzar Tab, MSD, Seoul, Korea, 50 mg), and Lasix (Lasix Tab, Handok, Seoul, Korea, 40 mg) were prescribed for the treatment of DCMP. He was discharged after improvement in his condition.
Thereafter, his cardiopulmonary symptoms progressed gradually during follow-up. Six months after discharge, he was diagnosed with persistent hemoptysis and dyspnea, and was re-admitted. Follow-up chest CT scans revealed interval progression of the cardiomegaly and pulmonary artery trunk enlargement (3.9 cm in diameter). The presence of focal GGO in the right middle lobe indicated aspirated blood. The BNP increased to 1398 pg/mL. Echocardiography showed aggravated systolic dysfunction (EF, 14%), mild pulmonary HTN, and plethora of the inferior vena cava. He received conservative treatment and was discharged after relief. Two months later, he was admitted to the emergency because of unconsciousness. On follow-up brain CT angiography, the previous right MCA aneurysm was slightly increased in diameter (5 mm) and was accompanied by a subarachnoid hemorrhage. Aneurysmal rupture was suspected, and an emergency operation was performed. At surgery, severe brain swelling was observed; however, clipping of the aneurysm was performed successfully. He was recovered without neurologic deficit.
ADPKD is one of the most commonly inherited renal diseases and systemic disorders, characterized by multiple, bilateral renal cysts with progressive enlargement of kidneys (1). Mutations in two genes, PKD1 and PKD2, of polycystic kidney disease have been known to be associated with ADPKD. PKD1 and PKD2 code for polycystin1 and 2 proteins, respectively. The localization of polycystins outside of renal tubules results in various extrarenal manifestations of ADPKD (2). Ultrasonography (US) is used for diagnosing ADPKD. US can detect cysts larger than 0.5 cm in diameter. In high-risk individuals with family history of ADPKD, at least three cysts (unilateral or bilateral) are needed for diagnosis in the age range 15-39 years. At least two and four cysts are the criteria for individuals in the age range 40-59 years and older than 60 years, respectively. CT and magnetic resonance imaging (MRI) can detect smaller simple cysts less than 1 cm and can be used as screening modality if cyst size is limited to ≥ 1 cm. CT is especially useful in the evaluation of renal complications in ADPKD such as kidney stones, cyst hemorrhage, infection, and suspicious masses. MRI is particularly helpful in evaluating complex renal cysts (5).
ICA is more common in patients with ADPKD than in the general population (4.0-11.7% vs. 1.0%) (6). Moreover, the risk of ICA rupture is higher in patients with ADPKD than in the general population. Griffin et al. (7) reported that the expression of polycystin proteins in the arterial vascular wall increases the risk of ICA formation. The screening for ICA can be performed by CT angiography or MRI with excellent accuracy in both modalities. Because a family history of ICA rupture is the only established risk factor for ICA rupture in patients with ADPKD, the indications for screening include a family history of ruptured ICA in ADPKD patients (6). Based on previous studies, screening for asymptomatic ADPKD patients without family history of ICA is not routinely recommended. However, since ADPKD patients without family history have increased risk of ICA relative to the general population, the patient should be aware of increased risk. Luciano and Dahl (2) suggested additional screening for patients preparing for major surgery, high risk occupation (pilot), and patients with anxiety about their status. However, whether the preventive treatment of ICA should be performed or/and method between endovascular coiling or aneurysmal clipping in patients with ADPKD is still controversial.
In our case, based on the laboratory findings and the clinical features, the CT findings corresponded to pulmonary edema, and the patient's hemoptysis was thought to result from a cardiac problem rather than respiratory etiologies. Elevated capillary hydrostatic pressure caused by pulmonary HTN can lead to the disruption of some or all layers of the alveolar-capillary unit (8). Therefore, in our case, the hemoptysis was thought to originate from the pulmonary microcirculation because of systolic dysfunction and pulmonary HTN caused by left heart failure. The diagnosis of DCMP is based on the LVE and systolic dysfunction with an EF < 50% (9). Ischemic heart disease, the most common cause of heart failure, should be excluded. Less common causes of DCMP include myocarditis, structural heart disease (congenital or valvular), neuromuscular disorders, toxic or metabolic causes, immune dysregulation, and familial. After exclusion of detectable causes, many cases (65%) were regarded as idiopathic DCMP (9). In our case, the patient had no evidence of ischemic heart disease, structural heart disease, autoimmune diseases, virus infections, family history of heart diseases, and no medication history. Thus, our case can be categorized as idiopathic DCMP. As to hereditary causes, many chromosomal loci have been identified to be associated with DCMP. However, a few studies have shown direct evidence linking ADPKD and DCMP (10). Torres (4) suggested the association between ADPKD and cardiomyopathy given that PKD genes are strongly expressed in cardiac myocytes. In a recent study by Paavola et al. (10) PKD2 mutant heart of a zebrafish model showed impaired intracellular calcium cycling, indicating a case of heart failure. Reviewing large clinical database literature indicated that idiopathic DCMP was found in 7 out of 307 patients with ADPKD type 1 and in 6 out of 67 patients with ADPKD type 2 in which the prevalence of idiopathic DCMP was over 200-folds than in the general population. Thus, we thought that idiopathic DCMP might be associated with ADPKD in the patient although further studies are needed.
For cardiac manifestations such as HTN, LVH, and valvular abnormalities in ADPKD patients, Luciano and Dahl (2) suggested routine BP follow-up for HTN and HTN control in hypertensive patients for preventing LVH. Imaging modalities for screening LVH or valvular abnormalities are reserved for patients with murmur or symptoms of heart failure. Although cardiac MRI could be used for screening cardiac abnormalities, echocardiography is currently preferred modality because of its cost-effectiveness. The screening of DCMP is not recommended in the current literature. However, if radiologists or clinicians are aware of various cardiac manifestations in ADPKD patients, they can suggest the possibility of cardiac involvement such as DCMP and recommend echocardiography on the detection of cardiomegaly in chest radiography or CT performed for other symptoms.
In conclusion, we reported the first case of ADPKD combined with ICA and DCMP together with hemoptysis and dyspnea. In addition to ICA, DCMP may be another cardiovascular manifestation of ADPKD when idiopathic DCMP is suspected in a patient with ADPKD, although a strong association between ADPKD and DCMP is still unclear.
Figures and Tables
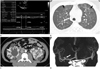
Fig. 1
Autosomal dominant polycystic kidney disease combined with intracranial aneurysm and dilated cardiomyopathy in a 26-year-old male patient.
A. M-mode view from parasternal long axis view of echocardiography shows abnormally increased LVIDd and LVIDs, suggesting marked LV dilation and global hypokinesia of LV wall. Calculated EF reveals severe systolic dysfunction (EF = 21%).
B. Lung window image of initial chest CT scan at level of aortic arch shows diffuse ground glass opacity with interlobular septal thickening in both lungs (arrows), suggesting pulmonary edema.
C. Contrast enhanced abdominal CT scan demonstrates numerous small and large renal cysts in both kidneys. Note a large cyst with wall calcification in the right kidney (arrow).
D. Maximal intensity projection image of initial brain CT angiography reveals a saccular aneurysm in the M1 segment of right MCA, measuring 4 mm (arrow).
Note.-d = diastole, EDV = end diastolic volume, EF = ejection fraction, ESV = end systolic volume, IVS = interventricular septum, LV = left ventricle, LVd Mass = left ventricular mass at end diastole, LVID = left ventricular internal diameter, LVPW = left ventricular posterior wall, RV = right ventricle, s = systole, SV = stroke volume, %FS = percent fractional shortening
References
1. Martinez JR, Grantham JJ. Polycystic kidney disease: etiology, pathogenesis, and treatment. Dis Mon. 1995; 41:693–765.
2. Luciano RL, Dahl NK. Extra-renal manifestations of autosomal dominant polycystic kidney disease (ADPKD): considerations for routine screening and management. Nephrol Dial Transplant. 2014; 29:247–254.
3. Handa SP. Cardiovascular manifestations of autosomal dominant polycystic kidney disease in young adults. Clin Invest Med. 2006; 29:339–346.
4. Torres VE. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1999; 34:xlv–xlviii.
5. Rahbari-Oskoui F, Mittal A, Mittal P, Chapman A. Renal relevant radiology: radiologic imaging in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2014; 9:406–415.
6. Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009; 5:221–228.
7. Griffin MD, Torres VE, Grande JP, Kumar R. Vascular expression of polycystin. J Am Soc Nephrol. 1997; 8:616–626.
8. Nauser TD, Stites SW. Diagnosis and treatment of pulmonary hypertension. Am Fam Physician. 2001; 63:1789–1798.
9. Parvari R, Levitas A. The mutations associated with dilated cardiomyopathy. Biochem Res Int. 2012; 2012:639250.
10. Paavola J, Schliffke S, Rossetti S, Kuo IY, Yuan S, Sun Z, et al. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J Mol Cell Cardiol. 2013; 58:199–208.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download