Abstract
A focal nodular hyperplasia (FNH) of the liver is the second most common benign hepatic tumor, but an interval growth or regression of FNH is uncommon. We report a case of spontaneous regression of FNH in a 34-year-old woman. This lesion was diagnosed during pregnancy and spontaneously regressed during a 5-year observation period after delivery.
A focal nodular hyperplasia (FNH) is a proliferation of normal non-neoplastic hepatocytes that is believed to be a local hyperplastic response to a vascular abnormality (1). It is the second most common benign hepatic tumor and most commonly incidentally found in woman in their 3rd and 4th decades of life (2). Female hormones have been suggested by clinical observations to play a role because of the female predomin-ance and the young age at onset (1, 3), but it is not clear whether there is an association between FNH and oral contraceptive (OC) or pregnancy. The natural history of FNH is still unknown, because long term follow-up studies of histologically proven FNH with conflicting results have been performed only in small series of patients (4, 5, 6). Herein, we report a patient with FNH of the liver, which was diagnosed during pregnancy and spontaneously regressed during a 5-year observation period after delivery.
A 34-year-old pregnant woman at 17 weeks and 3 days of gestation presented with hyperemesis gravidarum. The patient was multiparous (T3 P0 A1) and had no remarkable medical history. On admission, her physical examination was unremarkable except her pregnant state. Laboratory data including liver function test, hepatitis viral markers and tumor markers were within normal limits except a slightly elevated serum bilirubin level. The patient had no history of OCs use or estrogen therapy.
A transabdominal ultrasound examination demonstrated a 4 cm sized isoechoic solid mass in the IV segment of the liver. The color Doppler revealed blood flow around and within the mass. The initial differential diagnosis included FNH and hepatocellular adenoma. But it was not possible to perform contrast enhanced computed tomography (CT) or magnetic resonance imaging (MRI) because of her pregnant state.
At the 32th week of pregnancy, the patient underwent cesarean section due to refractory severe hyperemesis gravidarum. A dynamic abdominal CT was performed five days after the delivery. The pre-contrast CT scan showed a 4 cm sized, low density mass arising from segment IV of the liver. The mass was well enhanced on the arterial phase of CT scan. On the portal and delayed phases, the mass showed slightly low attenuation compared with the surrounding liver parenchyma. No central scar was noted on the CT images (Fig. 1).
A MRI was performed with a clinical 1.5-T unit (Signa HDx; GE Healthcare, Waukesha, WI, USA) with contrast enhancement (MultiHance; Bracco Diagnostics, Lake Success, NY, USA) (Fig. 2). It revealed a 4.2 × 4.0 cm sized mass with high signal intensity on T2-weighted image. The mass was intensely enhanced during the arterial phase except the central scar and was nearly isointense during the portal and delayed phases. On the hepatobiliary phase image acquired 2 hours after contrast injection, the mass was isointense except the hypointense central scar. An ultrasound-guided needle biopsy of the hepatic mass was performed at that time. The microscopic analysis confirmed the presence of fibrous septa, separating cirrhosis-like nodules of hepatocytes. And the fibrous septa contained medium to large thick-walled vessels. The diagnosis of FNH was made based on those findings. The patient was informed about this diagnosis and consented to follow-up conservatively.
The patient remained asymptomatic and was doing well. She underwent a contrast enhanced dynamic liver CT about 5 years after the initial diagnosis. On this follow-up study, the FNH had significantly regressed measuring 0.8 × 1.0 cm in size and the enhancement pattern of the tumor was not changed (Fig. 3).
FNH of the liver is a benign tumor-like lesion that is typically discovered by incidence during diagnostic imaging performed for other reasons. Some have speculated that hormones play a role in the pathogenesis of the tumor because FNH typically occurs in female of reproductive age and about 50-75% of patients with FNH are OCs users (2). Although endogenous or exogenous estrogens clearly play a role in the pathogenesis of hepatic adenoma, their relationship with focal nodular hyperplasia has been controversial discussed (5, 7). In addition, the course of FNH during pregnancy has not yet been studied in detail. But existing reports have mostly been showing a benign course of FNH during pregnancy; Mathieu et al. (5) described 12 patients with FNH that did not change in size during uneventful pregnancies. Cobey and Salem (8) summarized available data showing the growth progression of FNH during pregnancy in 1 out of 32 women only. Rifai et al. (9) described no substantial growth in 20 patients and no complications during pregnancy or birth in all 16 patients who responded to their questionnaire.
There have been conflicting results regarding the natural history of FNH. Although the majority of studies showed that an interval growth of FNH is uncommon, there are occasional reports of FNH cases with interval regression (5, 10). Mathieu et al. (5) followed 216 patients for 9 years and showed a size change of FNH in 4 of 136 patients only (3%). Among them, 3 cases showed interval regression and 1 showed interval growth.
In our case, the patient was diagnosed with FNH in pregnancy. Because the FNH was found incidentally during her pregnancy, we didn't know whether the pregnancy had influenced the FNH or not. At least, the size of the FNH was not changed on the follow-up CT images performed a few days after the delivery. The patient and her physician decided to conservatively follow-up because the FNH was confirmed on MRI and microscopic findings after the delivery. Also, the FNH regressed significantly during the 5-year follow-up after delivery. The patient had no history of exogenous estrogen use prior to the initial diagnosis of FNH and didn't have any therapy between her initial presentation and the last follow-up. There is neither sufficient data to conclude that the natural history of our patient's lesion can be attributed to her delivery nor is there sufficient data to conclude otherwise.
The correlation between the natural history of FNH and pregnancy/delivery remains unclear with some conflicting data and cases of postpartum long term follow-up are very rare. Therefore, further study is necessary to firmly establish the role of pregnancy and delivery in the natural history of FNH. A case-control or prospective cohort study with long term follow-up could potentially provide resolution.
Figures and Tables
 | Fig. 1A dynamic abdominal CT scan acquired five days after the delivery
A. Precontrast CT scan shows a 4.2 × 4.0 cm size, well defined exophytic growing low density mass arising from segment IV of liver.
B, C. On arterial phase, the mass is well enhanced (B), and shows slightly low attenuation compared with the surrounding liver parenchyma on portal phase (C).
|
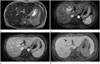 | Fig. 2Liver MRI using hepatocyte-specific contrast agent.
A. A T2-weighted imaging shows the high SI mass at segment IV.
B. Following intravenous administration of gadobenate dimeglumine, an arterial phase T1-weighted imaging (T1WI) shows an intensely enhancing mass with a nonenhancing central scar (arrow).
C. Portal phase image shows that the mass is isointense with the liver parenchyma and the central scar enhancing.
D. A hepatobiliary phase T1WI shows an isointense mass with a hypointense central scar (arrow).
|
References
1. Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985; 5:1194–1200.
2. Buetow PC, Pantongrag-Brown L, Buck JL, Ros PR, Goodman ZD. Focal nodular hyperplasia of the liver: radiologic-pathologic correlation. Radiographics. 1996; 16:369–388.
3. Côté C. Regression of focal nodular hyperplasia of the liver after oral contraceptive discontinuation. Clin Nucl Med. 1997; 22:587–590.
4. Di Stasi M, Caturelli E, De Sio I, Salmi A, Buscarini E, Buscarini L. Natural history of focal nodular hyperplasia of the liver: an ultrasound study. J Clin Ultrasound. 1996; 24:345–350.
5. Mathieu D, Kobeiter H, Maison P, Rahmouni A, Cherqui D, Zafrani ES, et al. Oral contraceptive use and focal nodular hyperplasia of the liver. Gastroenterology. 2000; 118:560–564.
6. Leconte I, Van Beers BE, Lacrosse M, Sempoux C, Jamart J, Materne R, et al. Focal nodular hyperplasia: natural course observed with CT and MRI. J Comput Assist Tomogr. 2000; 24:61–66.
7. Giannitrapani L, Soresi M, La Spada E, Cervello M, D'Alessandro N, Montalto G. Sex hormones and risk of liver tumor. Ann N Y Acad Sci. 2006; 1089:228–236.
8. Cobey FC, Salem RR. A review of liver masses in pregnancy and a proposed algorithm for their diagnosis and management. Am J Surg. 2004; 187:181–191.
9. Rifai K, Mix H, Krusche S, Potthoff A, Manns MP, Gebel MJ. No evidence of substantial growth progression or complications of large focal nodular hyperplasia during pregnancy. Scand J Gastroenterol. 2013; 48:88–92.
10. Halankar JA, Kim TK, Jang HJ, Khalili K, Masoom HA. Understanding the natural history of focal nodular hyperplasia in the liver with MRI. Indian J Radiol Imaging. 2012; 22:116–120.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download