This article has been corrected. See "Erratum: Comparative Analysis of Tuberculous Lymphadenitis and Kikuchi Disease of the Neck" in Volume 71 on page 101.
Abstract
Purpose
To compare the clinical and CT manifestations of Kikuchi disease (KD) and tuberculous lymphadenitis (TL).
Materials and Methods
111 patients with TL (55 men, 56 women, mean age 38.6 years, range 13-80 years) and 73 patients with KD (24 men, 49 women, mean age 26.8 years, range 8-61 years) were included in the study. Two observers independently compared sex, age, peripheral white blood cells, erythrocyte sedimentation rate (ESR), nodal distribution, nodal bilaterality, perinodal fat infiltration, muscle abscess, nodal conglomeration, necrotic and non-necrotic lymph nodes in the patients with TL and KD.
Results
KD patients showed a female predominance (67.1%). Patients with TL were older. Leukocytopenia and increased ESR were more frequent in patients with KD. KD more commonly affected lymph nodes in level II, III, and V, while TL more commonly involved lymph nodes in the upper paratracheal area. Perinodal fat infiltration was more frequent in KD. Muscle abscess was seen in patients with TL only (14%). Necrotic lymph nodes were more frequent in TL. A thin type was more frequent in TL.
Conclusion
KD showed female predominance, leukocytopenia, increased ESR, involvement of levels II, III, and V and frequent perinodal fat infiltration. TL patients were older than KD patients, were commonly affected in the upper paratracheal area, abscesses were shown only in this group and thin type necrotic lymph nodes were more frequent.
Kikuchi disease (KD) is a histiocytic necrotizing lymphadenitis with characteristic histological appearances and was first described by Kikuchi (1) in 1972. In general, the diagnosis of KD is made by evaluation of cervical lymphadenopathy (2).
Contrast-enhanced computed tomography (CT) is one of the best imaging techniques for evaluating neck mass (3) and KD is often confused with tuberculous lymphadenitis (TL) in the process of investigating cervical lymphadenopathy on CT. The specific clinical or radiological features of KD have not been fully clarified yet. Based on regional tendency, tuberculosis is more common in developing countries and it is now becoming common in developed countries with the spread of Acquired Immune Deficiency Syndrome (4). At first, KD was described only in Asians. However, KD has gradually been reported in many literatures on all races (5) and the clinical implementation of a differential diagnosis between the both diseases was required for an appropriate treatment. Anti-inflammatory treatment, steroids and immunosuppressive therapy have been used to control KD (6). In contrast, TL can be successfully treated with a six-month daily regimen of tuberculosis treatment (7). The characteristics of cervical lymph nodes affected by KD on neck CT have been reported in many literatures as follows; homogeneous lymph node enhancement, perinodal fat infiltration and an accentuation of the fascial planes of the neck (5, 8). Lymph node necrosis is fairly common in KD diagnosed at biopsy and the presence of necrosis can mimic TL, because caseous necrosis is one of the typical CT findings in TL. Therefore, it is important to study the characteristic CT findings of necrotizing lymphadenitis in TL and KD to ensure appropriate action (9) and to aid in the timely diagnosis of the diseases. Although CT findings of KD and TL have been described in few literatures, to our knowledge, there has been no large-scale analysis of the radiologic findings between the two diseases (10).
The purpose of our study was to compare the clinical and CT manifestations of KD and TL and to accurately differentiate and diagnose the two diseases.
The single institution retrospective study was conducted at the Soonchunhyang University Hospital and was approved by the Institutional Review Board of the institution. The requirement for informed consent was waived. This work was supported in part by the Soonchunhyang University Research Fund.
From September 2007 to December 2010, 111 patients with TL (55 men, 56 women, mean age 38.6 years, range 13-80 years) and 73 patients with KD (24 men, 49 women, mean age 26.8 years, range 8-61 years) were included in this study.
All CT scans were performed with 8 channel (GE lightspeed ultra; GE medical system, Milwaukee, WI, USA) or 64 channel (GE lightspeed VCT; GE medical system, Milwaukee, WI, USA) equipment. Scanning parameters were as follows: 1 table pitch, 120 kVp, 140 mAs, 5 mm thickness. After acquisition of unenhanced images, 100 mL of nonionic contrast material (Iomeron 350, Braco; Omnipaque 350, Nycomed/GE Healthcare, Milwaukee, WI, USA) was injected at 1.5 mL/s and followed by a 20 mL saline flush.
The neck CT was independently analyzed by two radiologists with 2 and 9 years of experience in interpreting head and neck CT on a Picture Archiving and Communication System (DeitViewer, Dongeun information technology Co. LTD, Cheonan, Korea).
We compared sex and age, peripheral white blood cells (normal range: 4000-10000/µL) and erythrocyte sedimentation rate (ESR) (normal range: less than 20 mm/hour) between TL and KD.
We reviewed nodal distribution, nodal bilaterality, perinodal fat infiltration, muscle abscess, nodal conglomeration and necrotic lymph nodes. We analyzed the CT manifestations of lymph nodes that were more than 1 cm in their short axes. Also the nodal distribution was evaluated in accordance to the imaging-based nodal classification recommended by Shah et al. (11).
Lymph nodes were divided into necrotic or non-necrotic. A necrotic lymph node was defined as having non-enhancing foci in the lymph node. We measured the Hounsfield unit (HU) using a manually defined circular region of interest (ROI) of approximately 4 mm2. At least, three ROI measurements were performed on the grossly lowest density area of each lymph node. Then the ROI values were averaged. A non-enhancing focus was present when there was less than 10 HU difference between the average HU value of the post-contrast and pre-contrast images. Non-necrotic lymph node included homogenous or heterogeneous enhancement without necrosis. The types of necrotic lymph nodes were categorized as thin or thick, depending on the proportion of the peripheral enhancement of the necrotic lymph node. A thin type was defined as a peripheral enhancing portion less than 2 mm thick and below 25% of the total area. A thick type was defined as a peripheral enhancing portion more than 2 mm thick or above 25% of the total area.
Since there is no gold-standard for necrotic lymph nodes, we performed an interobserver agreement test for necrosis, nodal bilaterality, nodal distribution and the type of necrotic lymph nodes.
Statistical analysis was performed using SPSS for Windows (version 14.0; SPSS Inc., Chicago, IL, USA). The chi-square test was used to compare following categorical variables: sex, leukocytopenia, leukocytosis, increased ESR, nodal distribution, perinodal fat infiltration, muscle abscess and nodal conglomeration. Independent samples t-test was used to compare following continuous variables: age, lymph node necrosis and type of necrotic lymph node. A p-value of < 0.05 indicated a statistically significant difference.
Interobserver agreement tests were performed using the two-way random-effects model to calculate the intraclass correlation coefficient (ICC) and the single kappa test was used to calculate the kappa value. The ICC was calculated for necrotic and non-necrotic lymph nodes. Our scale for the interpretation of ICC was adapted for the reproducibility as follows: less than 0.40 as poor; 0.40-0.60 as moderate; 0.61-0.80 as good and 0.81 as excellent. The kappa values were calculated for the two observers in assessing the following categorical variables: nodal distribution, nodal bilaterality, perinodal fat infiltration, muscle abscess and nodal conglomeration. Kappa values of 0-0.2 indicated slight agreement; 0.21-0.4 fair; 0.41-0.60 moderate; 0.61-0.8 substantial; 0.81 or greater excellent.
There was no significant gender difference in the TL patients. KD patients showed significant female predominance (67.1%) (p < 0.05). Patients with TL (38.6 ± 16.1 years) were older than those with KD (26.8 ± 10.5 years) (p < 0.05). The frequency of leukocytopenia and increased ESR were significantly higher in KD (p < 0.05) (Table 1).
TL more commonly affected lymph nodes in the upper paratracheal area (p < 0.05) as noted by both observers (Figs. 1, 2), while KD more commonly involved lymph nodes in level II, III, and V (Fig. 3). There was no significant difference in nodal bilaterality and nodal conglomeration between TL and KD. Perinodal fat infiltration was significantly higher in KD (Fig. 3). Muscle abscess was seen in TL only (14.4% in both observers, p < 0.05) (Fig. 2, Table 2). The degree of agreement was excellent in nodal bilaterality (k value = 1.0), muscle abscess (k value = 1.0) and perinodal fat infiltration (k value = 0.83). Good inter-observer agreement was seen in nodal conglomeration (k value = 0.71). The degree of agreement for nodal distribution was good in level II, III, and supraclavicular area, while moderate in level I, IV, V, VI, and paratracheal area.
Necrotic lymph nodes were more frequent in TL and non-necrotic lymph nodes were more frequent in KD. The thin type lymph node was more frequent in TL. There was no significant difference in the frequency of thick type between TL and KD (observer 1: p = 0.17, observer 2: p = 0.22). The degree of reliability was almost perfect in both necrotic (ICC value = 0.94) and non-necrotic (ICC value = 0.95) lymph nodes (Table 3).
KD and TL are very common in Asian countries and are often encountered during the evaluation of a cervical lymphadenopathy. Evolved KD in 2 or 3 weeks generally accompanies cervical lymphadenopathy in 56-98% of cases. In addition to lymphadenopathy, 30-50% of KD have mild fever associated with upper respiratory symptoms like TL (12).
The specific CT findings of KD are homogeneous lymph node enhancement with or without nodal necrosis and perinodal infiltration, while patients with TL usually have caseous central necrosis with peripheral rim enhancement with or without calcification. However, it is sometimes difficult to differentiate these diseases because they have overlapping findings on CT (13, 14).
Many previous studies reported a female to male ratio of KD about 1.1:1-2.75:1 (15, 16, 17). In our study, 67% of KD patients were female and the female to male ratio was 2:1. This result is in good agreement with previous studies. In our study, the female to male ratio in TL was 1:1. There is some controversy concerning the sex ratio in TL (18, 19). We suggest this disagreement may be due to several factors which include endemicity, human immunodeficiency virus co-epidemicity or the diverse biological, social and cultural variables between countries.
Schofer et al. (20) reported the age range of KD as 19 months to 75 years. Approximately 5-10% of affected patients were younger than 21 years, but KD rarely affected patients under 16 years of age. Primrose et al. (2) and Jayaraj et al. (21) reported that KD commonly affected young women with a peak age of incidence in the third decade of life, but rarely affected patients under 16 years of age. Chen et al. (22) reported that cervical TL occurred more commonly between 30 and 40 years of age. In our study, KD patients (mean age 26.8 years) were relatively younger than TL patients (mean age 38.6 years). This result was similar to previous reports.
In our study, 42.5% of KD patients showed leukocytopenia and it was more frequent than in TL (5.4%). Several authors reported that mild leukocytopenia was observed in 25-58% and leukocytosis in 2-5% of KD patients. Leukocytopenia in KD is related to the cytokine-mediated mechanisms of lymphadenitis (12, 17, 23, 24, 25).
Increased ESR was significantly more common in KD (43.8%) than in TL (20.7%). Hassan et al. (26) and Kuo (17) reported an possible association of leukocytopenia, atypical lymphocytosis and raised ESR with KD.
The updated nodal classification by Som et al. (27) further subclassified and subdivided cervical lymph nodes and we believed it would be hard to compare the two diseases due to the decreasing patient number in the subclassified groups. Instead, we used the classification by Shah et al. (11) to evaluate the lymph nodes in order to minimize confusing variables and to increase the statistical power.
Our study showed that KD affected in order of decreasing frequency the nodes in level V, II, III, IV, and I. This pattern was the same for both observers. Han et al. (28) reported that KD affected lymph nodes in level V and IV, II, III, and I and Kwon et al. (10) reported that KD in order of decreasing frequency were at levels II, V, III, and IV. Kato et al. (29) reported that cervical lymphadenopathy in KD showed predominantly unilateral nodal distribution in order of decreasing frequency at levels II, V, III, and IV. It appears that the frequency of level V and II lymph node involvement was higher than that of the other regions of the neck.
Our study showed that TL was seen in neck nodes order of decreasing frequency at level IV, V and in the supraclavicular area. This propensity was also the same for both observers. Iqbal et al. (30) and Choi et al. (14) showed that TL commonly involved lymph nodes in the posterior triangle and supraclavicular area. In our study, TL was more common in the upper paratracheal area (observer 1: 16.2%, observer 2: 15.3%) than in KD (observer 1: 5.5%, observer 2: 4.1%). Solak et al. (31) reported the paratracheal lymph node was the most frequent site of involvement in mediastinal TL. Kang et al. (32) reported the upper paratracheal lymph nodes as the most frequently involved area found in the chest CT of tuberculosis patients. Mycobacterium tuberculosis ingested by alveolar macrophages spreads through the lymphatic channels to regional hilar and mediastinal lymph nodes. Mycobacterium tuberculosis is an aerobic bacterium and is always found in the upper lung zone, resulting in a high frequency of TL in the highest mediastinum (19, 33).
In our study, perinodal fat infiltration was considerably more frequent in KD (observer 1: 64.6%, observer 2: 58.9%) than in TL (observer 1: 25.2%, observer 2: 30.6%). This is a common feature in KD, which may also be seen in lymphoma and metastasis. The mechanism of cell death in KD has been explained due to apoptosis induced by inflammatory mediators such as interferon and FasL. Interleukin-6 has also been reported to be increased during the acute phase of KD (12). Increased inflammatory mediators activate inflammatory cells including the mixtures of lymphoid cells and histiocytes and the characteristic karyorrhectic debris (10). Histologically, perinodal fat infiltration means that the surrounding structures around the lymph nodes are infiltrated by perivascular and interstitial inflammatory cells.
In the data of both observers was the necrotic lymph node significantly more common in TL than in KD. In addition, the thin type was significantly more present in TL than in KD. These might be explained by following: the pathologic feature of KD is characterized by a sub-acute coagulation necrosis with apoptosis while that of TL is characterized by early caseous necrosis with tissue destruction by the organism (12, 17, 24, 34).
In our study, muscle abscess was present in 16 patients with TL (14%, in both of two observers). There was an adjacent fat infiltration in 12 of 16 muscle abscess cases. The muscle abscess with adjacent fat infiltration might be due to the process of collar stud abscess formation. In tuberculosis, softened caseous necrotic lymph node can rupture and result in ulceration or a sinus with the adjacent deep fascia, and in turn, may lead to abscess formation in the muscle (35).
There was no significant difference in nodal bilaterality and nodal conglomeration between TL and KD in both observers (Table 2).
There were some limitations in our study. First, selection bias might have occurred because imaging analysis was done only on the lymph nodes which were more than 1 cm in their short diameter. Second, lymph nodes more than 1 cm in the short diameter did not concur with the histopathologic diagnosis. This may have led to limitations in obtaining exact predictive values. Third, since a small ROI was used for the measurement of necrotic areas in an affected lymph node, the possibility of technical error could not be excluded. Fourth, there was moderate interobserver agreement in terms of nodal distribution in level I, IV, and V. We believe it was influenced by vague anatomical boundaries on axial CT images. Improvements in interobserver agreements may be attained by using multiplanar reformatted and three-dimensional multidetector CT images.
In summary, our study showed younger age, female predominance, leukocytopenia, increased ESR, frequent nodal distribution at level II, III, and V, perinodal fat infiltration and non-necrotic lymph node as valuable factors to differentiate KD from TL. On the other hand, nodal distribution favoring the upper paratracheal area, presence of muscle abscess, necrotic lymph node and thin type were valuable factors in diagnosing TL.
In conclusion, KD and TL can be differentiated from each other using clinical data and CT manifestations before histopathologic confirmation.
Figures and Tables
Fig. 1
Tuberculous lymphadenitis in a 15-year-old girl who presented with palpable right neck mass a few days ago.
A-C. Axial CT images show multiple necrotic lymph nodes, bilaterally, in level II, III, IV, and V. The thin type necrotic lymph nodes at level II of left neck (white arrow) shows peripheral enhancing portion less than 2 mm thick and below 25% of the total area of lymph node.
D. The necrotic lymph node is at the right paratracheal area (black arrow). Excisional biopsy was performed at level II of the right neck and the histopathologic diagnosis was consistent with tuberculosis.

Fig. 2
Tuberculous lymphadenitis in a 34-year-old man who presented with painful swelling on the right neck one week ago. Axial image shows a large abscess splitting the right platysma muscle (white arrow), suggesting a muscle abscess. Adjacent subcutaneous fat infiltration (arrowheads) was also noted. Tuberculosis was confirmed by ultrasound guided fine needle aspiration of the abscess.
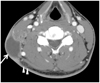
Fig. 3
Kikuchi disease in a 23-year-old man who presented with palpable mass in the right neck 3 days ago. Axial images show multiple necrotic and non-necrotic lymph nodes at level II, III, IV, and V bilaterally.
A. There are conglomerated homogenous enhancing lymph nodes (white arrow) with perinodal fat infiltration (arrowhead) at level II of left neck.
B. The thick type necrotic lymph node at level III of the left neck (black arrow) has peripheral enhancing portion more than 2 mm thick and above 25% of total lymph node area.
C, D. There are another non-necrotic lymph nodes at level III, IV, and V which show left side predominancy. Ultrasound-guided fine needle aspiration biopsy was performed on the level II lymph node of the left neck. Histopathologic diagnosis was Kikuchi's lymphadenopathy.

References
1. Kikuchi M. Lymphadenitis showing focal reticulum cell hyperplasia with nuclear debris and phagocytes: a clinicopathological study. Acta Hematol Jpn. 1972; 35:379–380.
2. Primrose WJ, Napier SS, Primrose AJ. Kikuchi-Fugimoto disease (Cervical Subacute Necrotising Lymphadenitis): an important benign disease often masquerading as lymphoma. Ulster Med J. 2009; 78:134–136.
3. Schwetschenau E, Kelley DJ. The adult neck mass. Am Fam Physician. 2002; 66:831–838.
4. Bennie MJ, Bowles KM, Rankin SC. Necrotizing cervical lymphadenopathy caused by Kikuchi-Fujimoto disease. Br J Radiol. 2003; 76:656–658.
5. Ahuja A, Ying M, Yuen YH, Metreweli C. Power Doppler sonography to differentiate tuberculous cervical lymphadenopathy from nasopharyngeal carcinoma. AJNR Am J Neuroradiol. 2001; 22:735–740.
6. Altuntas F, Sari I, Canoz O, Yildiz O, Eser B, Cetin M, et al. Kikuchi-Fujimoto disease: a rare but important cause of fever and lymphadenopathy in pregnant women. Am J Hematol. 2006; 81:118–120.
7. Campbell IA, Ormerod LP, Friend JA, Jenkins PA, Prescott RJ. Six months versus nine months chemotherapy for tuberculosis of lymph nodes: final results. Respir Med. 1993; 87:621–623.
8. Na DG, Chung TS, Byun HS, Kim HD, Ko YH, Yoon JH. Kikuchi disease: CT and MR findings. AJNR Am J Neuroradiol. 1997; 18:1729–1732.
9. Song JY, Cheong HJ, Kee SY, Lee J, Sohn JW, Kim MJ, et al. Disease spectrum of cervical lymphadenitis: analysis based on ultrasound-guided core-needle gun biopsy. J Infect. 2007; 55:310–316.
10. Kwon SY, Kim TK, Kim YS, Lee KY, Lee NJ, Seol HY. CT findings in Kikuchi disease: analysis of 96 cases. AJNR Am J Neuroradiol. 2004; 25:1099–1102.
11. Shah JP, Strong E, Spiro RH, Vikram B. Surgical grand rounds. Neck dissection: current status and future possibilities. Clin Bull. 1981; 11:25–23.
12. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004; 122:141–152.
13. Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007; 26:50–54.
14. Choi EC, Moon WJ, Lim YC. Case report. Tuberculous cervical lymphadenitis mimicking metastatic lymph nodes from papillary thyroid carcinoma. Br J Radiol. 2009; 82:e208–e211.
15. Jha BC, Dass A, Nagarkar NM, Gupta R, Singhal S. Cervical tuberculous lymphadenopathy: changing clinical pattern and concepts in management. Postgrad Med J. 2001; 77:185–187.
16. Castro DJ, Hoover L, Castro DJ, Zuckerbraun L. Cervical mycobacterial lymphadenitis. Medical vs surgical management. Arch Otolaryngol. 1985; 111:816–810.
17. Kuo TT. Kikuchi's disease (histiocytic necrotizing lymphadenitis). A clinicopathologic study of 79 cases with an analysis of histologic subtypes, immunohistology, and DNA ploidy. Am J Surg Pathol. 1995; 19:798–809.
18. Fontanilla JM, Barnes A, von Reyn CF. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis. 2011; 53:555–562. World Health Organization. Global tuberculosis control annual reports from 1997 to 2008. Geneva, Switzerland: WHO, 2008. Available at: http://www.who.int/tb/publications/en/. Accessed on: April 20, 2008.
19. Schofer JM, Tong TC, Tanen DA. Kikuchi's disease: a rare cause of cervical lymphadenitis and fever. J Emerg Med. 2005; 29:151–153.
20. Jayaraj SM, Lloyd J, Frosh AC, Patel KS. Kikuchi-Fujimoto's syndrome masquerading as tuberculosis. J Laryngol Otol. 1999; 113:82–84.
21. Chen YM, Lee PY, Su WJ, Perng RP. Lymph node tuberculosis: 7-year experience in Veterans General Hospital, Taipei, Taiwan. Tuber Lung Dis. 1992; 73:368–371.
22. Parappil A, Rifaath AA, Doi SA, Pathan E, Surrun SK. Pyrexia of unknown origin: Kikuchi-Fujimoto disease. Clin Infect Dis. 2004; 39:138–143.
23. Lin HC, Su CY, Huang CC, Hwang CF, Chien CY. Kikuchi's disease: a review and analysis of 61 cases. Otolaryngol Head Neck Surg. 2003; 128:650–653.
24. Felgar RE, Furth EE, Wasik MA, Gluckman SJ, Salhany KE. Histiocytic necrotizing lymphadenitis (Kikuchi's disease): in situ end-labeling, immunohistochemical, and serologic evidence supporting cytotoxic lymphocyte-mediated apoptotic cell death. Mod Pathol. 1997; 10:231–241.
25. Hassan M, Anees A, Zaheer S. Kikuchi-fujimoto disease: diagnostic dilemma and the role of immunohistochemistry. J Clin Med Res. 2009; 1:244–246.
26. Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000; 174:837–844.
27. Han HJ, Lim GY, Yeo DM, Chung NG. Kikuchi's disease in children: clinical manifestations and imaging features. J Korean Med Sci. 2009; 24:1105–1109.
28. Kato H, Kanematsu M, Kato Z, Teramoto T, Kondo N, Hirose Y, et al. MR imaging findings of cervical lymphadenopathy in patients with Kikuchi disease. Eur J Radiol. 2011; 80:e576–e581.
29. Iqbal M, Subhan A, Aslam A. Papillary thyroid carcinoma with tuberculous cervical lymphadenopathy mimicking metastasis. J Coll Physicians Surg Pak. 2011; 21:207–209.
30. Solak O, Sayar A, Metin M, Erdoğu V, Cuhadaroğlu S, Turna A, et al. The coincidence of mediastinal tuberculosis lymphadenitis in lung cancer patients. Acta Chir Belg. 2005; 105:180–182.
31. Kang SJ, Kim YH, Jung CY, Lee HJ, Hyun MC. Clinical characteristics and radiologic patterns of adelescents with pulmonary tuberculosis: relevance to the reactive tuberculosis. Pediatr Allergy Respir Dis. 2012; 22:163–170.
32. Leung AN. Pulmonary tuberculosis: the essentials. Radiology. 1999; 210:307–322.
33. Sarwar A, Haque AU, Aftab S, Mustafa M, Moatasim A, Siddique S, et al. Spectrum of Morphological Changes in Tuberculous Lymphadenitis. Int J Pathol. 2004; 2:85–89.
34. Teo SY, Ong CL. Clinics in diagnostic imaging (108). Tuberculous dactylitis of the thumb, mediastinal and left hilar lymphadenopathy, and probable left cervical lymphadenopathy. Singapore Med J. 2006; 47:243–249. quiz 250.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download