Abstract
Hypertensive encephalopathy typically presents with bilateral parietooccipital vasogenic edema. Brainstem and cerebellar edema are uncommon in association with typical supratentorial changes. We experienced three cases of atypical hypertensive encephalopathy involving brainstem and cerebellum as well as cerebral white matter, which showed characteristic alternating linear bright and low signals in the pons, the so-called "stripe sign". We report these cases here with a brief literature review.
Hypertensive encephalopathy is an acute disorder characterized by severe hypertension, headache, vomiting, altered mental status, visual changes, and seizures. Syndromes of hypertensive encephalopathy are usually reversible if quickly diagnosed and treated. The most common finding of hypertensive encephalopathy is bilateral edema in the supratentorial white matter, especially in the parieto-occipital area. However, atypical feature of hypertensive encephalopathy involving the frontal lobes, brainstem, cerebellum, cortical watershed zones, and basal ganglia has been sporadically reported. Brainstem and/or cerebellar lesions may be presented as the only abnormalities in unusual cases (1). Here we report three cases of hypertensive encephalopathy involving brainstem showing characteristic alternating linear bright and low signals in the pons on T2-weighted magnetic resonance imaging (MRI), although many brainstem lesions showed no peripheral sparing compared to those of osmotic demyelination (2). This retrospective study has been approved by our Institutional Review Board. The requirement for obtaining informed consent from patients was waived.
A 36-year-old woman suffered from headache and dizziness one week before her admission. She had a previous history of poorly controlled hypertension without antihypertensive medication. On admission, she showed alert mental status. Blood pressure was of 270/170 mm Hg with body temperature of 36℃. Neurological examination revealed no abnormal finding. Hematologic examination showed increased blood levels of urea nitrogen (29.3 mg/dL) and creatinine (2.53 mg/dL). Urine examination revealed proteinuria. Her blood electrolyte level and cerebrospinal fluid study were within the normal limits.
Brain computed tomography (CT) and MRI were performed immediately after her arrival. CT showed diffuse low density areas in the midbrain, pons, cerebellum, and periventricular white matter. MRI after the CT on the same day revealed extensive hyperintensity in the whole midbrain, pons and upper medulla on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images. We also observed scattered and irregular shaped lesions in the cerebellum, bilateral thalami and bilateral periventricular cerebral white matter. Alternating linear bright and low signals were found on T2-weighted and FLAIR images without peripheral sparing in the pons (Fig. 1A, B). There was no mass effect. These lesions showed increased apparent diffusion coefficient (ADC) value (Fig. 1C) as slight hypointense without enhancement on T1-weighted images. Magnetic resonance (MR) angiography was normal. She immediately received intravenous infusion of a calcium channel blocker (nicardipine hydrochloride, 40 mg) which controlled her systemic pressure to 140-170/70-90 mm Hg. Antihypertensive medications were followed by an oral calcium channel blocker (nifedipine, 66 mg) and an angiotensin II receptor blocker (candesartan cilexetil, 16 mg). Two days after the admission, her elevated blood pressure was consistently reduced to the normal range with disappearance of headache and dizziness. She was discharged on the 11th day post-admission with prescribed antihypertensive medication. Her lesions were completely resolved within 3 weeks.
A 42-year-old man admitted to our hospital with a history of headache for three months. One week before the admission, he developed bilateral blurred vision and gait disturbance. He was diagnosed with hypertension about two years ago. However, he was not treated with any medication.
Neurologic examination revealed that he was normal with alert metal status. Physical examination revealed his blood pressure at 236/170 mm Hg with body temperature of 36.3℃. Funduscopic examination revealed suspicious retinal microhemorrhage. His blood creatinine and electrolyte levels were normal. Cerebrospinal fluid study showed normal finding except mildly elevated white blood cell count of 1/mm3 and protein concentration of 79.7 mg/dL (normal: 12-60 mg/dL).
MRI revealed extensive hyperintensity in the brainstem, thalamus and cerebellum on T2-weighted and FLAIR images. We also observed the alternating bright and low signals in the pons as seen in case 1. The pons and cerebellum appeared swollen with compression of the fourth ventricle and mild obstructive hydrocephalus. These lesions showed increased ADC value with no enhancement. There was no peripheral sparing of the pons. After 6 months of antihypertensive treatment, his headache and blurred vision improved. There was no available follow-up image.
A 55-year-old woman was presented to our hospital with headache and vomiting. She visited a local hospital previously with high arterial blood pressure. Although she received oral medication for arterial hypertension, she suffered progressive headache after that. Her metal state was alert. There was no abnormal finding on neurologic or funduscopic examinations. Her blood pressure was at 215/109 mm Hg with body temperature of 36.8℃ on admission. Her blood creatinine and blood electrolyte levels were normal. Cerebrospinal fluid evaluation was not performed.
MRI showed extensive hyperintensity in the brainstem, cerebellum and periventricular deep cerebral white matter on T2-weighted and FLAIR images (Fig. 2). We also observed the symmetric T2-hyperintensity in the pons with stripes as seen in case 1 and 2. Tendency of the peripheral sparing was observed in the pons. ADC maps revealed increased diffusion in the brainstem lesion. Multiple microbleedings were presented in the cerebellum, suggesting hypertensive microangiopathy. There was no evidence of abnormal enhancement in the brain parenchyma. MR angiography revealed no abnormality. After 2 weeks of antihypertensive treatment, her headache subsided. Follow-up MRI showed complete disappearance of abnormal hyperintense lesions.
T2-hyperintensity lesions are uncommonly seen in the brainstem, cerebellum, or basal ganglia in hypertensive encephalopathy. In our three cases, the main MR finding was hyperintensity on T2-weighted image in the brainstem and cerebellum with little or considerable supratentorial deep white matter involvement. Patient 3 showed peripheral sparing of pontine lesion known as typical pattern in osmotic demyelination.
Characteristic MRI findings of pontine lesions revealed alternating linear bright and low signals in all our three cases, probably signifying fluid between the transverse pontine bundles (3). We defined this specific pattern of the pons as the "stripe sign". A similar finding was described in osmotic demyelination syndrome that showed symmetric triangular-shaped T2-hyperintensity with stripes in the central pons (4). The transverse pontine fibers were seen as lines of preserved brain passing from one side to the other. In literatures, osmotic demyelination syndromes show the sparing of peripheral pons and pontine portion of corticospinal tracts. However, hypertensive encephalopathy does not. Acute lesions might reveal restricted diffusion in osmotic demyelination (4).
We reviewed 17 articles on hypertensive encephalopathy with brainstem involvement for the evaluation of either presence or absence of the stripe sign and other indicative MR findings. A total of 26 patients were reviewed in 17 articles. Two patients were excluded due to no images given. Of the remaining 24 cases, the stripe sign was shown in 5 patients on MRI images and 2 cases with descriptions. However, the stripe sign was not shown or mentioned in other cases. It has been widely accepted that hypertensive brainstem encephalopathy does not spare the peripheral pons (4). However, the periphery of pons was spared in 8 of 24 cases. Only one case by Bhagavati et al. (1) showed mild obstructive hydrocephalus by brainstem swelling. Diffusion weighted imaging (DWI) was evaluated in 15 cases by either imaging or literature. All 15 cases had normal DWI, suggesting vasogenic edema (Table 1). In our cases, case 3 revealed sparing of the peripheral pons whereas case 2 showed mass effect by brainstem swelling.
Hypertensive encephalopathy involving brainstem should be differentiated from acute infarction, central pontine myelinolysis, brainstem glioma, acute disseminated encephalomyelopathy, and infectious encephalitis. Acute infarction could be ruled out by the absence of acute ischemic findings on initial DWI and resolution of MR finding with a recovery of clinical symptom. Acute disseminated encephalomyelopathy, infectious encephalitis, and central pontine myelinolysis could be differentiated readily based on laboratory examination and rapid recovery. Glioma involves expansion and mass effect. The presence of stripe sign in the pons without obvious mass effect or contrast enhancement could be used to exclude the possibility of glioma, encephalitis, and demyelinating disease.
Pathophysiologic changes of hypertensive encephalopathy is associated with vasogenic edema without evidence of cytotoxic edema or infarction. Vessels in posterior circulation are sparsely innervated by sympathetic nerves, therefore poorly initiating vasoconstriction in response to suddenly increased arterial pressure (5). Deep regions such as thalamus, basal ganglia, and brainstem are supplied from the arterioles branches of the middle cerebral artery or basilar artery. Cortex and subcortex are supplied from terminal branch arteries (6). Deep gray matter and brain stem are affected by more intense arterial blood pressure than the cerebral cortex and subcortex. Therefore, severe and lasting acceleration of elevated arterial pressure may contribute greatly to the involvement of deep structures. Our cases showed particular MR pattern of the hypertensive encephalopathy, thus allowing a better understanding of the changes associated with arterial hypertension.
We concluded that the presence of diffuse T2-hyperintensity with stripe sign in the pons in patients with severe paroxysmal hypertension could be helpful for the diagnosis of hypertensive encephalopathy. Tumor or inflammatory conditions could be easily excluded by their non-enhancing nature. However, the peripheral sparing of pontine lesions on T2-weighted image can occur in both osmotic demyelination syndrome and hypertensive encephalopathy.
Figures and Tables
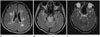 | Fig. 1A 36-year-old female presented with headache. MR images showed hyperintensity involving the periventricular cerebral white matter (A), pons and cerebellum bilaterally, with the stripe sign in the pons on fluid-attenuated inversion recovery (FLAIR) images (B). Corresponding apparent diffusion coefficient map revealed increased diffusion in the brainstem (C). Follow-up MR images 3 weeks after initial MRI showed significant diminution of hyperintensity on FLAIR images. |
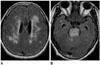 | Fig. 2A 55-year-old female suffered from headache and vomiting. MR images showed symmetric hyperintensity in the periventricular cerebral white matter (A), brainstem and cerebellum on fluid-attenuated inversion recovery images with the stripe sign in the pons. The periphery of the pons was relatively spared (B). |
References
1. Bhagavati S, Chum F, Choi J. Hypertensive encephalopathy presenting with isolated brain stem and cerebellar edema. J Neuroimaging. 2008; 18:454–456.
2. Ono Y, Manabe Y, Hamakawa Y, Murakami T, Omori N, Hayashi Y, et al. Localized lesions on MRI in a case of hypertensive brainstem encephalopathy. Intern Med. 2005; 44:1002–1005.
3. de Seze J, Mastain B, Stojkovic T, Ferriby D, Pruvo JP, Destée A, et al. Unusual MR findings of the brain stem in arterial hypertension. AJNR Am J Neuroradiol. 2000; 21:391–394.
4. Osborn AG. Acquired Metabolic and Systemic Disorders. In : Osborn AG, editor. Osborn's Brain: Imaging, Pathology, and Anatomy. Salt Lake City, UT: Amirsys;2012. p. 907–957.
5. Beausang-Linder M, Bill A. Cerebral circulation in acute arterial hypertension--protective effects of sympathetic nervous activity. Acta Physiol Scand. 1981; 111:193–199.
6. Sadoshima S, Fujii K, Yao H, Kusuda K, Ibayashi S, Fujishima M. Regional cerebral blood flow autoregulation in normotensive and spontaneously hypertensive rats--effects of sympathetic denervation. Stroke. 1986; 17:981–984.
7. Karasawa S, Kawanami T, Kimura H, Kurita K, Kato T. An unusual case of hypertensive encephalopathy involving the brain stem. Intern Med. 2004; 43:448–449.
8. Kanazawa M, Sanpei K, Kasuga K. Recurrent hypertensive brainstem encephalopathy. J Neurol Neurosurg Psychiatry. 2005; 76:888–890.
9. Gamanagatti S, Subramanian S. Hypertensive encephalopathy: isolated pons involvement mimicking central pontine myelinolysis. Korean J Radiol. 2006; 7:218–219.
10. Uchino M, Haga D, Nomoto J, Mito T, Kuramitsu T. Brainstem involvement in hypertensive encephalopathy: a report of two cases and literature review. Eur Neurol. 2007; 57:223–226.
11. Kang SY, Choi JC, Kang JH. Two cases of hypertensive encephalopathy involving the brainstem. J Clin Neurol. 2007; 3:50–52.
12. Shintani S, Hino T, Ishihara S, Mizutani S, Shiigai T. Reversible brainstem hypertensive encephalopathy (RBHE): clinicoradiologic dissociation. Clin Neurol Neurosurg. 2008; 110:1047–1053.
13. Chang GY, Keane JR. Hypertensive brainstem encephalopathy: three cases presenting with severe brainstem edema. Neurology. 1999; 53:652–654.
14. Yasuda Y, Akiguchi I, Imai T, Sonobe M, Kage M. Hypertensive brainstem encephalopathy. Intern Med. 2003; 42:1131–1134.
15. Nagata M, Maeda M, Tsukahara H, Maier SE, Takeda K. Brain stem hypertensive encephalopathy evaluated by line scan diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004; 25:803–806.
16. Doi Y, Kimura F, Fujiyama T, Fujimura C, Nishina T, Sato T, et al. Hypertensive brainstem encephalopathy without parieto-occipital lesion--two case reports. Neurol Med Chir (Tokyo). 2006; 46:75–79.
17. Yoshida K, Yamamoto T, Mori K, Maeda M. Reversible posterior leukoencephalopathy syndrome in a patient with hypertensive encephalopathy--case report. Neurol Med Chir (Tokyo). 2001; 41:364–369.
18. Kumai Y, Toyoda K, Fujii K, Ibayashi S. Hypertensive encephalopathy extending into the whole brainstem and deep structures. Hypertens Res. 2002; 25:797–800.
19. Cruz-Flores S, de Assis Aquino Gondim F, Leira EC. Brainstem involvement in hypertensive encephalopathy: clinical and radiological findings. Neurology. 2004; 62:1417–1419.
20. Park JH, Kim SM, Shin HW, An SJ. Hypertensive brainstem encephalopathy involving deep supratentorial regions: does only blood pressure matter? Neurol Int. 2010; 2:e9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download