Abstract
Purpose
The aim of this study was to investigate and report magnetic resonance imaging (MRI) findings of iatrogenic spinal infection (ISI).
Materials and Methods
We retrospectively reviewed the clinical and MRI findings on 18 patients diagnosed with ISI. The MRI findings were evaluated for the number of spinal segments showing inflammation in the longitudinal span and affected vertebral bodies and discs, the presence of abscess in the epidural or paravertebral space, paravertebral myositis, and skip lesions.
Results
Among the 18 patients, the range of the longitudinal span of spinal inflammation was 2-11 (mean = 5.84) vertebral segments. 17 of the 18 patients had three or more contiguous vertebral segments. The osteomyelitis and disc destruction was apparent in 77.8% and 66.7% of the patients, and 78% of patients with osteomyelitis showed involvement of one or two vertebrae; 91.7% of patients with disc destruction showed involvement of single disc. The incidence of epidural or paravertebral abscesses, and paravertebral myositis were 88.9%, and 94.4%. There were no spinal skip lesions.
There are many types of interventional pain management, including acupuncture, epidural pain block, selective nerve root block, facet joint block, percutaneous vertebroplasty, micro-invasive procedures, and others. Pain management can reduce the length of hospitalization and avoid risks inherent in surgery. Accordingly, interventional pain management is increasing relied upon. Although post-procedural infection is rare, incidences are likely to increase as interventional pain management becomes more common (1,2,3). A complicating factor is that preexisting pain and discomfort might mask its symptoms. Moreover, the proximity of some post-procedural complications including iatrogenic spinal infection (ISI) to the central nervous system can leave various and serious sequelae, including death (4,5,6,7,8). For these reasons, post-procedural infection demands close scrutiny. However, its diagnosis remains difficult . Although magnetic resonance imaging (MRI) modality has been used for evaluating infections of the spine and soft tissues (9), there are no published MRI findings on ISI following various pain management procedures. Therefore, the objective of this study was to investigate and report MRI findings on ISI following various pain management procedures in order to facilitate its early diagnosis.
This study was approved by the Institutional Review Board. Between January 2003 and March 2011, we retrospectively reviewed medical records of 1907 individuals who had undergone lumbosacral-spine MRI or whole-spine MRI. Of those, 90 who had developed infectious spondylitis were chosen. We defined iatrogenic infectious spondylitis as developing infections after recent pain management procedures in the spinal region. Exclusion criteria were: patients who had ineligible MRI results (e.g., the lack of axial scan or enhancement), patients whose infections were at location other than that of the interventional pain management, and patients whose infections were incurred after open surgery. Finally, 18 patients with the diagnosis of ISI were included in this study, including clinicoradiological diagnosis for four patients and histopathological diagnosis for 14 patients based on the isolation of causative organisms at the site of spinal involvement (percutaneous specimens in eight patients, and surgical specimens in six patients). The 18 patients included ten men and eight women with average age of 64.3 years (range, 47-80 years) (Table 1).
The presenting symptoms included aggravated or persistent lower back pain with radiating pain to the lower legs (n = 13, 72.2%), fever (n = 4, 22.2%), general weakness (n = 2, 11.1%), pain in the spinal region that received the pain management procedure (n = 1, 5.56%), or stuporous mental status (n = 1, 5.56%) Laboratory findings in almost all cases were indicative of infectious spondylitis. However, three (16.7%) patients showed normal levels of inflammatory markers, such as white blood cell counts, erythrocyte sedimentation rate, and C-reactive protein. Twelve (67%) patients suffered from preexisting diseases, including diabetes (n = 9, 50%), dilated cardiomyopathy (n = 2, 11.1%), angina (n = 2, 11.1%), chronic liver disease (n = 1, 5.56%), nephrotic syndrome (n = 1, 5.56%), chronic obstructive pulmonary disease (n = 1, 5.56%), gout (n = 1, 5.56%), and Parkinson's disease (n = 1, 5.56%). Of the 12 patients, three had more than one underlying disease. Pertinent pain management included the use of needles (n = 9, 50%), acupuncture (n = 6, 33.3%), epidural anesthesia (n = 2, 11.1%), and percutaneous laser discectomy (n = 1, 5.56%). Symptoms were observed at mean 18.2 (range, 1 to 40) days after the pain management procedure. The mean interval time between the pain management procedure and MRI was 28.3 (range, 10 to 60) days. Main organisms involved in the infections were Staphylococcus (n = 12, 66.6%) (Table 2).
Nine patients were cured with antimicrobial treatment. One patient was cured by percutaneous abscess drainage. Eight patients underwent surgical drainage, including four who required revision operation (Table 3). In three patients, no culture grew from surgical specimens although histopathologic reports were well correlated with findings of spinal infections.
MRI was performed using 1.5-T superconducting systems (Signa and Genesis Signa, General Electric Medical Systems, Milwaukee, WI, USA). Our standard protocol for spinal MRI was as follows: unenhanced fast spin-echo T1-weighted images [T1WI, repetition time (TR)/echo time (TE), 416-600/10-15 msec]; fast spin-echo T2-weighted images (T2WI, TR/TE, 3500-4000/80-127 msec); post-contrast axial, sagittal and coronal fat-suppressed T1WI (TR range/TE range, 450-716/9-16 msec). Images were obtained after IV administration of gadolinium-based contrast agent. Imaging parameters used for image enhancement were similar to those employed in unenhanced imaging. Typical MRI parameters were: field of view at 18 cm for axial plane and 28 cm for sagittal plane; number of excitations at 2; matrix size at 320 × 224 in case of enhanced axial T1-weighted MR images; slice thickness at 4 mm; intersection gap at 5-10 mm; echo-train length at 2-28.
Two experienced musculoskeletal radiologists evaluated the images. Their final conclusions were reached by consensus. The following MRI findings were analyzed: the number of spinal segments spanned; the number of osteomyelitis lesions in the vertebral bodies; the presence of intervertebral disc destruction; subligamentous inflammation; abscesses in the epidural or paravertebral space; myositis in the dorsal and ventral paravertebral space, and skip lesions. The number of spanned spinal segments was counted as the number of involved vertebral levels from T10 to S3. The presence of homogeneous low-signal intensity within the vertebra on T1WI, confirmed on contrast-enhanced images was considered evidence of bone marrow involvement (9). Intervertebral disc involvement was suspected when poorly defined margins and fluid-like signal intensities between the disc and adjacent subchondral bone marrow space were identified on T1WI and T2WI (10). Intervertebral disc destruction was defined as breaching of the subchondral cortex by extension of the infectious lesion through the endplate into the disc with loss of disc height on sagittal images (11). Fluid-like signal intensity from the anterior and posterior aspects of the affected vertebral bodies was considered indicative of subligamentous spread of infection. Abscess was identified by reviewers based on contrast-enhanced images. Fluid-like signal intensity in the muscles was considered as indicative of myositis. Paravertebral space was categorized into two groups: dorsal space (spinalis thoracis, multifidus, longissimus, iliocostalis) and ventral space (psoas and quadratus lumborum). Skip lesions were identified based on axial and sagittal images.
Among the 18 patients, the mean range of the longitudinal span of infection was 5.84 (range, 2 to 11) vertebral segments. All patients except one had abscess or myositis and osteomyelitis or discitis involving three or more contiguous vertebral segments in the longitudinal axis (Table 4, Fig. 1). Fourteen (78%) patients showed involvement of one or two vertebral bodies (Fig. 2). Twelve (67%) patients had intervertebral disc destruction. Two (11%) patients with vertebral involvement had intact intervertebral discs (Fig. 3). Eleven (61%) patients had single disc involvement. Four (22%) had intact vertebral bodies and intervertebral discs despite large abscesses and prominent myositis (Fig. 4). Thirteen (72%) patients showed subligamentous extensions of infection. Fifteen (83%) and fourteen (78%) patients had abscesses in the epidural and paravertebral space, respectively. Fourteen (78%) and fifteen (83%) patients showed myositis in the dorsal and ventral paravertebral spaces, respectively. No patient had skip lesions.
It was reported that ISI resulting from treatment procedures involving the spine could occur in vertebral bodies, intervertebral discs, paravertebral soft tissues, and epidural space, very rarely involving the contents of spinal canal or spinal cord (12, 13). The incidence of ISI has risen recently due to the increasing use of lumbar-region pain management procedures such as nerve blocks, epidural injections, catheters, as well as oriental-medicine such as acupuncture, cupping, and moxibustion (14, 15, 16, 17, 18). Regardless of the type of pain management procedure, all practices carry associated risk of post-procedural infection or ISI. Despite improvements in diagnostic accuracy, antibiotic therapy, surgical technique, and postoperative care, infectious complications following pain management procedures have resulted in significant morbidity and occasional mortality (4, 5, 6, 7, 8, 19). The incidence of ISI following various pain management procedures in all community-acquired infections was not reported in our survey. To our knowledge, there have been only a few case reports on imaging findings pertaining to ISI following various pain management procedures (18, 19, 20, 21, 22, 23, 24). Reports on MRI findings are very rare, even though MRI has been reported to be particularly useful for early detection of spinal infection.
The most common risk factors of ISI are mainly related to insufficient aseptic conditions during medical procedures. In the present study, 83.3% of patients received alternative pain management procedures involving needles or and/or acupuncture in a doctor's examination room whereas 16.7% received epidural anesthesia and percutaneous laser discectomy in operating room. It has been reported that ISI is more prevalent in patients with diabetes mellitus, immune deficiency, or chronic debilitating conditions caused by alcohol or drug addiction (5, 6, 15, 17, 21, 23). Among our 18 patients, 66.7% had preexisting diseases, including the most common predisposing factor diabetes mellitus. Hence, when performing pain management procedures on such patients, clinicians should consider the potential of infection.
The mean time interval between the procedure and symptom onset was 18.2 (range, 1 to 40) days. Literature reviews (8, 15) revealed that the mean time interval between the procedure and symptom onset were 5 (range, 1 to 60) days or 9.75 (range, 2 to 20) days. In this study and earlier investigations (8, 15), symptoms and signs were nonspecific. Ordinary laboratory findings were of little help for differential diagnosis of ISI following various pain management procedures from other non-iatrogenic infections. Staphylococcus aureus, the most commonly identified skin-resident organism and etiologic agent of iatrogenic spinal or epidural abscesses (5, 6, 7, 8, 14, 15, 19, 21, 22, 23), was found in 78.6% of our culture-positive patients. These findings suggested that most cases of ISI were caused by patient's endogenous floral seeding through contaminated needles or failure of sterilization techniques. Treatment of ISI following various pain management procedures should be initiated as soon as possible. While microbiologic reports are pending, standard antibiotic regimens with staphylococcal coverage could be administered.
Contrary to the typical findings for community-acquired pyogenic spondylitis, seventeen (94.4%) of the 18 patients showed wide longitudinal span of infection involving more than three vertebral segments. Classic MRI finding on pyogenic spondylitis is obvious inflammations in most of the bodies of two adjacent vertebrae and the intervening intervertebral disc. In our cases, the mean number of osteomyelitic vertebral bodies was 1.67, which was less than the 2.3-3 in community-acquired pyogenic spondylitis or tuberculous spondylitis (10, 25). Disc destruction was noted in 66.7% of our patients, which was less frequent than previously reported. Six of our patients did not show involvement of two adjacent vertebrae or the intervening intervertebral disc. In contrast to community-acquired pyogenic spondylitis, involvement of vertebral bodies and intervertebral disc was relatively weak or faint, but with larger epidural or paravertebral abscesses and extensive myositis in the dorsal and ventral paravertebral spaces similar to tuberculous spondylitis (10, 25, 26). Epidural abscess was noted in 88.9% of our patients, which was significantly more frequent than what was usually observed in community-acquired pyogenic spondylitis (27, 28). Spinal epidural abscess usually occurs secondarily to hematogenous dissemination from distant foci in the body. In some cases, it is directly disseminated via infected urinary tract or spinal osteomyelitis. Or, as in our cases, spinal epidural abscess also occurred via direct inoculation during pain management procedures. The incidence of epidural abscess is increasing when predisposing factors such as chronic debilitation, immune-suppressed status or prior spine surgery become more common. Myositis and abscesses in the dorsal and ventral paravertebral spaces were also common. Following various pain management procedures, ISI appeared to commence at the injection site or along the tract of injection to spread more widely in the spinal canal or paravertebral soft tissues due to the absence of physical barrier. Therefore, direct inoculation of infecting organisms might be liable to produce extensive soft-tissue infection and rapid dissemination, which could lead to life-threatening situations if not treated promptly. Subligamentous extension was noted in 72.2% of our patients. Such frequency was similar to that of tuberculous spondylitis reported previously (10, 25, 26). None of our patients showed skip lesions.
In conclusion, following various pain management procedures, diagnosis of ISI is rare. Early diagnosis is essential to the prevention of serious morbidity and mortality. MRI imaging findings of wide longitudinal span of infection, involvement of no more than two vertebral bodies and a single disc, large abscesses, extensive myositis, and no skip lesions can be useful for the diagnosis of ISI following various pain management procedures.
Figures and Tables
Fig. 1
Iatrogenic spinal infection following laser discectomy for L5-S1 with aggravated back pain in 55-year-old man.
A. Sagittal fat-suppressed enhanced T1-weighted image shows diffuse enhancement in L5 and S1 vertebral bodies with anterior and posterior subligamentous extensions. Large multiloculated abscesses in the epidural space (arrowheads) extend longitudinally from the L3 to S2.
B. Axial fat-suppressed enhanced T1-weighted image at the level of L5-S1 shows large multiloculated abscesses in the epidural space (arrowheads) and paravertebral space (arrows) with thin, smooth walls. In the muscles, inflammatory changes, including psoas, iliacus, multifidus, longissimus, and gluteus, are evident.
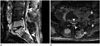
Fig. 2
Iatrogenic spinal infection following epidural anesthesia with febrile illness and back pain in 69-year-old woman.
A. Sagittal MR images (T2-weighted, T1-weighted, fat-suppressed contrast-enhanced) show involvement of two vertebral bodies and one disc with disc destruction. Inflammatory changes are shown along the route of injection in the interspinous region of L3-4 (arrow). The longitudinal 10-vertebral-segment span of infection extends vertically from T11 to S3 (not shown here).
B. Axial fat-suppressed enhanced T1-weighted image shows extensive myositis in the dorsal (thin arrows) and ventral (thick arrows) paravertebral spaces with multiple abscesses.
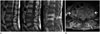
Fig. 3
Iatrogenic spinal infection following acupuncture with aggravated pain and fever in 74-year-old woman.
A. Sagittal MR images (T2-weighted, T1-weighted, fat-suppressed contrast-enhanced) show involvement of three vertebral bodies (L4, L5, and S1, arrows) without involvement of intervening intervertebral discs. The longitudinal 11-vertebral-segment extends vertically from T10 to S3 (not shown here). Image distortion by metal artifacts in the L4 and L5 vertebrae from previous, eight-years-earlier spinal surgery is shown.
B. Axial fat-suppressed enhanced T1-weighted image at the level of L4-5 shows large paravertebral abscess (thick arrow) and pyomyositis in the dorsal paravertebral space (thin arrows).

Fig. 4
Iatrogenic spinal infection following acupuncture with aggravated pain in 48-year-old woman. Sagittal MR images (T2-weighted, T1-weighted, fat-suppressed contrast-enhanced) show non-involvement of vertebral body or intervening intervertebral disc despite large epidural (arrowheads) and paravertebral (arrow) abscesses and wide longitudinal span of infection. The L5 body shows a chronic compression fracture and normal marrow signal intensity.
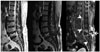
Table 1
Clinical Summary of 18 Patients with Iatrogenic Spinal Infection

Note.-Alternative Tx = the name of pain management procedures entailing the use of needles is uncertain, COPD = chronic obstructive pulmonary disease, DCMP = dilated cardiomyopathy, DM = diabetes, LBP = aggravated or persistent low back pain, Px-MR = period between the given procedure and the first MR study, Px-Sx = period between the given procedure and the onset of presenting symptoms, RP = radiating pain to both lower legs, SD = syndrome
References
1. Epstein NE. The risks of epidural and transforaminal steroid injections in the Spine: commentary and a comprehensive review of the literature. Surg Neurol Int. 2013; 4:Suppl 2. S74–S93.
2. McGrath JM, Schaefer MP, Malkamaki DM. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011; 12:726–731.
3. Zeidman SM, Thompson K, Ducker TB. Complications of cervical discography: analysis of 4400 diagnostic disc injections. Neurosurgery. 1995; 37:414–417.
4. Trautmann M, Lepper PM, Schmitz FJ. Three cases of bacterial meningitis after spinal and epidural anesthesia. Eur J Clin Microbiol Infect Dis. 2002; 21:43–45.
5. Sillevis Smitt P, Tsafka A, van den Bent M, de Bruin H, Hendriks W, Vecht C, et al. Spinal epidural abscess complicating chronic epidural analgesia in 11 cancer patients: clinical findings and magnetic resonance imaging. J Neurol. 1999; 246:815–820.
6. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000; 23:175–204. discussion 205.
7. Schütze M, Piek J. Paracervical abscesses as life-threatening complications of outpatient pain treatment. Report of three cases. Neurosurg Focus. 2004; 17:E13.
8. Gaul C, Neundörfer B, Winterholler M. Iatrogenic (para-) spinal abscesses and meningitis following injection therapy for low back pain. Pain. 2005; 116:407–410.
9. Modic MT, Feiglin DH, Piraino DW, Boumphrey F, Weinstein MA, Duchesneau PM, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985; 157:157–166.
10. Hong SH, Choi JY, Lee JW, Kim NR, Choi JA, Kang HS. MR imaging assessment of the spine: infection or an imitation? Radiographics. 2009; 29:599–612.
11. Lee SW, Lee SH, Chung HW, Kim MJ, Seo MJ, Shin MJ. Candida spondylitis: comparison of MRI findings with bacterial and tuberculous causes. AJR Am J Roentgenol. 2013; 201:872–877.
12. Greenspan A. Orthopedic imaging: a practical approach. 5th ed. Philadelphia: Lippincott Williams & Wilkins;2011. p. 789–819.
13. Resnick D, Niwayama G. Osteomyelitis, septic arthritis, and soft tissue infection: the axial skeleton. In : Resnick D, editor. Diagnosis of bone and joint disorders. Philadelphia, PA: WB Saunders;2002. p. 2481–2509.
14. Xu S, Wang L, Cooper E, Zhang M, Manheimer E, Berman B, et al. Adverse events of acupuncture: a systematic review of case reports. Evid Based Complement Alternat Med. 2013; 2013:581203.
15. Kindler CH, Seeberger MD, Staender SE. Epidural abscess complicating epidural anesthesia and analgesia. An analysis of the literature. Acta Anaesthesiol Scand. 1998; 42:614–620.
16. Pobiel RS, Schellhas KP, Pollei SR, Johnson BA, Golden MJ, Eklund JA. Diskography: infectious complications from a series of 12,634 cases. AJNR Am J Neuroradiol. 2006; 27:1930–1932.
17. Alcock E, Regaard A, Browne J. Facet joint injection: a rare form cause of epidural abscess formation. Pain. 2003; 103:209–210.
18. Lee JH, Cho JH, Jo DJ. Cervical epidural abscess after cupping and acupuncture. Complement Ther Med. 2012; 20:228–231.
19. Kim SY, Han SH, Jung MW, Hong JH. Generalized infection following facet joint injection -A case report-. Korean J Anesthesiol. 2010; 58:401–404.
20. Weingarten TN, Hooten WM, Huntoon MA. Septic facet joint arthritis after a corticosteroid facet injection. Pain Med. 2006; 7:52–56.
21. Park MS, Moon SH, Hahn SB, Lee HM. Paraspinal abscess communicated with epidural abscess after extra-articular facet joint injection. Yonsei Med J. 2007; 48:711–714.
22. Orpen NM, Birch NC. Delayed presentation of septic arthritis of a lumbar facet joint after diagnostic facet joint injection. J Spinal Disord Tech. 2003; 16:285–287.
23. Byun YS, Kim HT, Chang SA, Lee SR, Hwang DH, Kim SH. Iatrogenic spinal infection after injection therapy in spine. J Korean Soc Spine Surg. 2006; 13:299–305.
24. Lindner A, Warmuth-Metz M, Becker G, Toyka VV. Iatrogenic spinal epidural abscesses: early diagnosis essential for good outcome. Eur J Med Res. 1997; 2:201–205.
25. Kim SY, Hong SJ, Lee CY, Chung KB, Park CM. Tuberculous spondylitis vs pyogenic spondylitis: focusing on the discriminative MR findings for differentiation. J Korean Radiol Soc. 2007; 56:183–189.
26. Jung NY, Jee WH, Ha KY, Park CK, Byun JY. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. AJR Am J Roentgenol. 2004; 182:1405–1410.
27. Kapeller P, Fazekas F, Krametter D, Koch M, Roob G, Schmidt R, et al. Pyogenic infectious spondylitis: clinical, laboratory and MRI features. Eur Neurol. 1997; 38:94–98.
28. Cheung WY, Luk KD. Pyogenic spondylitis. Int Orthop. 2012; 36:397–404.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download