Abstract
Tube tract implantation metastasis of non-small cell lung cancer is an extremely rare complication of surgical pericardiotomy. We report a case of tube tract seeding along the previous chest tube tract in the subxiphoid region. The subxiphoid tube tract seeding was created during pericardial window operation of a 48-year-old male patient with lung cancer for the drainage of malignant pericardial effusion.
Surgical pericardiotomy is an effective method to treat malignant pericardial effusion. Although pericardiotomy is generally considered safe, it has its risk of complications, including hemothorax, arrhythmia, hypotension, or heart failure (1). Metastatic tumor seeding along a transient indwelling drainage tube is an extremely rare complication of pericardiotomy. To our knowledge, no such case has ever been reported. We present a case of tumor implantation along the pericardiomtomy tube tract in a patient with lung cancer.
A 48-year-old man was admitted to hospital due to dyspnea that had started a few days before. The patient was diagnosed with non-small cell lung cancer (adenocarcinoma) in the left lower lobe with brain and bone metastases in May 2012, which subsequently progressed despite 3 courses of chemotherapy with various chemotherapeutic regiments [Gefitinib (Iress®), May 2012 through November 2012; Pemetrexed (Alimta®)-cisplatin, December 2012 through January 2013; Erlotinib (Tarceva®), May 2013 through July 2013]. Chest radiograph taken at the time of admission showed cardiomegaly with a moderate amount of left-sided pleural effusion. Electrocardiography showed low voltage sinus tachycardia (mean, 115/min). Transthoracic echocardiography revealed a large amount of pericardial effusion causing hemodynamic compromise compatible with cardiac tamponade. The patient underwent a pericardial window operation on the day of admission. About 2500 mL of bloody pericardial effusion was drained. Two chest tubes (one via the subxiphoid region and the other via the lateral chest wall) introduced though separate stab incisions were connected to a suction machine (Fig. 1A). Both chest tubes were left in place for 5 days. The pericardial specimen was submitted for histological analysis which revealed fibrosis with chronic inflammation and vascular proliferation. The pericardial fluid was submitted for cytological analysis which revealed metastatic adenocarcinoma. The patient's condition improved considerably after the procedure with no further complications.
Chest CT performed 12 days after the surgery revealed new soft tissue density nodules along the previous chest tube tract in the subxiphoid region (Fig. 1B). Although the nodules were not biopsied, repeated chest CT performed 1 month later showed a significant increase in nodule size with progression of nodular thickening of pericardium (Fig. 1C). The patient received chemotherapy with a new regimen. Chest CT performed at 3-month after the pericardial window operation revealed marked regression of both the subxiphoid nodules and the pericardial thickenings (Fig. 1D).
Malignant pericardial effusion is a common and serious manifestation in malignancies. Accessible treatment options include simple drainage and surgical approach, with the primary goal to relieve symptoms and improve quality of life. In emergency cases, with cardiac tamponade or significant effusion, initial relief could be obtained with percutaneous pericardiocentesis, occasionally followed by drainage with indwelling catheter or thoracic surgery (pericardiotomy or pericardial window operation) (1).
The most serious complication of pericardiocetesis is injury to the myocardium or coronary vessels. In addition, pneumothorax, hemothorax, serious arrhythmia, and puncture of the peritoneal cavity or abdominal organ can also occur. Chylopericardium and intrapericardial thrombosis have rarely been reported (2). A large echocardiography-based study reported that the incidence of major complications after pericardiocentesis was between 1.3% and 1.6% (3). Tract metastasis is an extremely rare complication of pericardiocentesis or pericardiotomy (4), where as catheter-tract metastasis is relatively common (0.5% in three large series of a combined 374 cases) complication of malignant pleural effusion (5). The occurrence of implantation metastasis has been reported in other interventional or surgical procedures, such as fine needle lung biopsy, percutaneous transhepatic biliary drainage, percutaneous ethanol injection for the treatment of hepatocellular carcinoma or liver metastasis, and gastrostomy tube insertion and laparoscopy (6, 7).
The mechanism of implantation metastasis is uncertain. Possible mechanisms of tumor cell seeding include tumor cell dissemination into soft tissue during a procedure and tumor cell growth along a tract as in perineural tumor growth (7). Possible risk factors of tumor dissemination have been postulated by many authors. Tumor dissemination may depend on the caliber and the type of the device used, the number of procedures performed, the absence of normal parenchyma overlying the lesion, and the skill of operator. Of those risk factors, the type of the device used seems to be most important. The larger the caliber of the device is used, the greater the opportunity for tumor cells to seed along the tract (4). The duration between a percutaneous procedure and the appearance of an implantation lesion is highly variable, from 2 to 26 months (8). In most cases, time to tumor development is shorter in procedures using more aggressive techniques (4). In our patient, only 12 days elapsed after the chest tube insertion before the seeding nodules appeared on CT. Such a rapid tumor growth may have been related to the large caliber of the indwelling chest tube or the aggressive nature of the tumor as evidenced by rapid interval enlargement of pericardial nodule (Fig. 1B, C), lung nodules, and mediastinal lymph nodes.
The limitation of the current report is the lack of pathologic confirmation. In principle, various conditions such as infection could be considered as differentials. However, although placement of a pericardial indwelling catheter was known to carry a risk of infection (9), there were no infection signs either at that time of the imaging or during the cytotoxic chemotherapy. In addition, the soft tissue at the previous tube tract progressed and regressed simultaneously with the metastatic pericardial nodules, pulmonary nodules, and mediastinal lymphadenopathies before and after the chemotherapy, respectively. Although there was no histological proof on nodules or pericardial thickenings, cytological metastasis revealed that fluid flowed through both regions. Therefore, it was very likely that the soft tissue represented tumor implantation.
In conclusion, we report a case of lung cancer implantation along the previous pericardiotomy tube tract as an extremely rare complication of malignant pericardial effusion treatment.
Figures and Tables
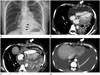 | Fig. 1Chest PA taken immediately after pericardial window operation. Two indwelling chest tubes are inserted via subxiphoid (arrows) and intercostal routes, respectively (A). Chest CT obtained 12 days after surgery reveals linear arrayed soft tissue density nodules along the tract of the subxiphoid chest tube (removed) (B). CT obtained 1 month after surgery reveals remarkable interval growth of the seeded nodules and progression of pericardial nodular thickening (C). Post-chemotherapy CT (3 months after surgery) shows marked regression of the seeded nodules and the pericardial thickenings (D). |
References
1. Burazor I, Imazio M, Markel G, Adler Y. Malignant pericardial effusion. Cardiology. 2013; 124:224–232.
2. Ceron L, Manzato M, Mazzaro F, Bellavere F. A new diagnostic and therapeutic approach to pericardial effusion: transbronchial needle aspiration. Chest. 2003; 123:1753–1758.
3. Tsang TS, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002; 77:429–436.
4. Quecedo E, Febrer I, Martínez-Escribano JA, Navarro-Iváñez R, Aliaga A. Tumoral seeding after pericardiocentesis in a patient with a pulmonary adenocarcinoma. J Am Acad Dermatol. 1994; 31(3 Pt 1):496–497.
5. Janes SM, Rahman NM, Davies RJ, Lee YC. Catheter-tract metastases associated with chronic indwelling pleural catheters. Chest. 2007; 131:1232–1234.
6. Al-Saif OH, Sengupta B, Meshikhes AW. Port site metastases after a laparoscopic abdominoperineal resection of rectal cancer: report of a case. Surg Today. 2011; 41:412–414.
7. Loew R, Dueber C, Schwarting A, Thelen M. Subcutaneous implantation metastasis of a cholangiocarcinoma of the bile duct after percutaneous transhepatic biliary drainage (PTBD). Eur Radiol. 1997; 7:259–261.
8. Kim JH, Kim YT, Lim HK, Kim YH, Sung SW. Management for chest wall implantation of non-small cell lung cancer after fine-needle aspiration biopsy. Eur J Cardiothorac Surg. 2003; 23:828–832.
9. Uramoto H, Hanagiri T. Video-assisted thoracoscopic pericardiectomy for malignant pericardial effusion. Anticancer Res. 2010; 30:4691–4694.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download