Abstract
Osmotic demyelination syndrome (ODS), an acquired demyelinating condition of the central pons and/or other regions of the brain, is frequently associated with rapid correction of hyponatremia. There are several reports of ODS in other clinical setting such as malnutrition, alcoholism, transplantation, malignancy, and chronic debilitating illness. However, cases of ODS associated with chemotherapy have not been frequently reported. Here, we describe a case of ODS in a normonatremic patient recently underwent chemotherapy for colon cancer. The diagnosis was confirmed by MRI showing a typical T2 hyperintensity in the central pons. This case suggests that ODS is not always associated with hyponatremia and that ODS can have a favorable clinical and radiologic prognosis.
Osmotic demyelination syndrome (ODS), an acquired demyelinating disease of the central pons, was first described in 1959 by Adams et al. (1) in hyponatremic alcoholics. This disorder was formerly known as central pontine myelinolysis, extrapontine myelinolysis, or a combination of both (2). ODS has been classically defined as a symmetrical and selective destruction of myelin sheaths in the central portion of the basis pontis that typically occurs after rapid correction of hyponatremia (1, 3). However, ODS may develop in other medical conditions such as electrolyte imbalance, syndrome of inappropriate antidiuretic syndrome (SIADH), chronic renal failure, organ transplantation, and chemotherapy (3). Patients typically have various symptoms, ranging from mild neurologic symptoms to a complete locked-in syndrome, coma, or death (1,2,3). In this paper, we describe a case of ODS in a patient with normonatremia who recently received chemotherapy for colon cancer. We also performed relevant literature review on this disease.
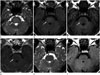
A 66-year-old man presented to our hospital with a 1 month history of intermittent headache. There was no prior history of any neurological disorder. He underwent low anterior resection for colon cancer and adjuvant chemotherapy 5 years ago. One month ago, he received a palliative chemotherapy with 5-fluorouracil, oxaliplatin and leucovorin. After starting the chemotherapy, he had intermittent headaches in the right temporal area. Although he received medical treatment for tension headache, there was no improvement for his headache. He had no other diseases such as chronic renal disease, hypertension, or diabetes mellitus. Physical examination and laboratory studies revealed no gross abnormalities. A careful neurological examination revealed no focal sign. Therefore, magnetic resonance (MR) imaging was performed to evaluate intracranial lesion. MR imaging showed T2 hyperintense lesion in the central pons without mass effect. This lesion revealed hypointensity on T1-weighted imaging without contrast enhancement. There were no other abnormalities in the rest of the brain (Fig. 1A-D). The prepontine cistern and fourth ventricle were unaffected by this lesion. There was no evidence of electrolyte abnormalities (sodium at 137 mmol/L, potassium at 4.1 mmol/L, chloride at 97 mmol/L, calcium at 9.2 mg/dL). The patient gradually improved without any specific treatment. After 1 month, headache disappeared. Moreover, a 2-month follow-up brain MR imaging with gadolinium showed a complete resolution of the preexisting pontine lesion (Fig. 1E, F). Therefore, the patient was diagnosed as ODS.
ODS is an uncommon demyelinating disorder of unknown cause. Although there was no mention of electrolyte imbalance in the first description of ODS by Adams et al. (1), ODS was well described as a complication of rapid hyponatremia correction in many previous reports (4). Other factors associated with ODS include alcohol abuse, malnutrition, chronic debilitating diseases, individual predisposition, dehydration, osmolarity fluctuations, liver dysfunction, SIADH, chemotherapy, and prolonged diuretic use (5).
The pathogenesis of ODS is still under debate. Histological studies have shown that oligodendrocytes are susceptible to osmotic stresses, especially in high-density brain areas, such as the central pons, thalamus, cortex, putamen, lateral geniculate bodies, and other extrapontine sites (4, 5). Myelinolysis in ODS is due to cellular stress resulting from fluctuating osmotic forces and ion shifts, leading to changes in cell volume and cell membrane function. Other clinical studies have shown that myelinolysis in ODS maybe caused by excess of sodium in the brain during correction of hyponatremia or compression of myelin by edematous cellular elements (6). However, unlike other demyelinating diseases, the neurons and axons are typically preserved in ODS with no inflammatory reaction (7).
Computed tomography (CT) is less sensitive than MR imaging in depicting ODS. CT finding reveals symmetric hypoattenuating lesion in the basis pontis and extrapontine regions without definite mass effect. MR imaging shows a characteristic T2 hyperintensity in the central pons, usually sparing the ventral pons. On T1-weighted imaging, the lesion is hypointensity without enhancement after the administration of contrast material. Diffusion-weighted MR imaging is the most sensitive imaging modality which could detect the lesion within 24 hours of development of symptoms. The severity of the lesion is not correlated with the severity of symptoms. As clinical symptoms subside, the lesion will decrease in size and signal intensity on T2-weighted and fluid-attenuated inversion recovery imaging (8).
In the present case, ODS occurred in a patient with normonatremia who received a palliative chemotherapy for colon cancer. Our patient complained a nonspecific pattern of headache without neurologic deficits. We considered a list of differential diagnosis including infarction, focal pontine leukoencephalopathy, atypical posterior reversible encephalopathy syndrome, brainstem encephalitis, tumor; brainstem glioma or atypical metastasis, and multiple sclerosis. However, these were excluded in this case based on the unifocal nature of the lesion, sparing of the peripheral pons, the lack of mass effect, the lack of significant contrast enhancement and complete resolution after 2 months. In addition, the patient was clinically stable without any neurological signs or symptoms related to brainstem pathology. Based on clinical features and MRI imaging results, we concluded that ODS was the most likely diagnosis.
There were only few cases of ODS associated with chemotherapy used for underlying malignancy. However, there is no literature explaining an exact mechanism of ODS in patient with chemotherapy, prevalence of chemotherapy-induced ODS, or associated chemoagents. Yau et al. (9) reported two cases of hyponatremia-related ODS in patients with nasopharyngeal carcinoma after chemotherapy using cisplatin. Hyponatremia is a common therapy complication of cisplatin. Dolciotti et al. (3) reported that ODS occurred in a normonatremic patient with colon cancer after chemotherapy using 5-fluorouracil and oxaliplatin.
In conclusion, this report suggests that the role of malignancy in ODS should be considered because it may be more commonly observed in clinical practices than suggested in literature. Although there are only a few case reports of normonatremic ODS in patients with malignancy and the cause of chemotherapy-related ODS are under debate, we suggest that clinical awareness of this disorder is very important for accurate diagnosis and management.
Figures and Tables
Fig. 1
A 66-year-old man presented with a 1 month history of intermittent headache.
A, B. Axial T2-weighted (A) and fluid-attenuated inversion recovery (FLAIR) (B) MR images reveal a hyperintense lesion in the central pons without mass effect (arrows).
C, D. Axial T1-weighted MR image (C) demonstrates T1 hypointensity in the central pons, and axial contrast enhanced T1-weighted MR image (D) shows no definite enhancement of the lesion (arrows).
E, F. On follow-up MR imaging after 2 months, the central pontine lesion shows a complete resolution on axial T2-weighted (E) and FLAIR (F) images.
References
1. Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959; 81:154–172.
2. Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry. 2004; 75:Suppl 3. iii22–iii28.
3. Dolciotti C, Nuti A, Cipriani G, Borelli P, Baldacci F, Logi C, et al. Cerebellar ataxia with complete clinical recovery and resolution of MRI lesions related to central pontine myelinolysis: case report and literature review. Case Rep Neurol. 2010; 2:157–162.
4. King JD, Rosner MH. Osmotic demyelination syndrome. Am J Med Sci. 2010; 339:561–567.
5. Huq S, Wong M, Chan H, Crimmins D. Osmotic demyelination syndromes: central and extrapontine myelinolysis. J Clin Neurosci. 2007; 14:684–688.
6. Lupato A, Fazio P, Fainardi E, Cesnik E, Casetta I, Granieri E. A case of asymptomatic pontine myelinolysis. Neurol Sci. 2010; 31:361–364.
7. Cramer SC, Stegbauer KC, Schneider A, Mukai J, Maravilla KR. Decreased diffusion in central pontine myelinolysis. AJNR Am J Neuroradiol. 2001; 22:1476–1479.
8. Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011; 23:369–374.
9. Yau TK, Yiu HY, Lee WM. Central pontine myelinolysis: report of two occurrences after cisplatin-containing chemotherapy for nasopharyngeal carcinoma. Clin Oncol (R Coll Radiol). 1993; 5:395–339.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download