INTRODUCTION
Vestibular schwannoma, a benign tumor of vestibulocochlear nerve arising from the Schwann cells, is also known as uncommon cause of hearing loss (1, 2). Vestibular schwannoma is usually hypovascular. Even a large tumor of vestibular schwannoma can be totally resected without major morbidity. However, unusual hypervascular vestibular schwannoma is often complicated by excessive tumor bleeding. Therefore, preoperative diagnosis of hypervascular vestibular schwannoma is important for the preparation of operation risks (3, 4).
We experienced a surgically proven hypervascular vestibular schwannoma in a 52-year-old woman. Here we describe the imaging findings of the hypervascular vestibular schwannoma case, with an emphasis on the hypervascularity of the tumor.
CASE REPORT
A 52-year-old female was admitted to our hospital for an evaluation of a 3-month history of decreased left hearing power. She had no significant medical history.
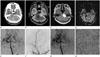
On enhanced brain CT, an intracranial extra-axial left cerebellopontine angle (CPA) mass was noted. The lesion was a lobulating-contoured heterogeneously enhancing solid mass at about 3.1 × 3.2 × 2.2 cm in size. On early arterial phase imaging (Fig. 1A), intratumoral and peritumoral enhancing vascularities were visible. Mild mass effect was seen causing compression of left aspect of pons and the fourth ventricle. On delayed enhanced brain CT, heterogeneously strong enhancement of the tumor was observed. T2-weighted images revealed hyperintensity of the tumor with intratumoral and peritumoral signal void structures (Fig. 1B). Enhanced T1-weighted images showed inhomogeneous strong enhancement of the mass with focal internal auditory canal portion (Fig. 1C). The CPA tumor showed dramatically increased relative cerebral blood volume (rCBV) on perfusion MRI, implying very hypervascularity (Fig. 1D). Cerebral angiography revealed early tumor staining supplied by left middle meningeal artery and left anterior inferior cerebellar artery (Fig. 1E, F). Early venous drainage was detected (Fig. 1G).
The patient underwent a subtotal excision of the tumor. On the surface of tumor, prominently engorged vessels distributing to the tumor were observed. Hematoxylin and eosin staining of tumor showed strongly stained nuclear palisading and hyaline vessel walls. These were histopathologic features of vestibular schwannoma (Fig. 1H).
The patient was discharged in stable condition after 10 days of hospital stay. We made a plan to have elective multi-staged surgery later due to the risk of tumor bleeding.
DISCUSSION
Vestibular schwannomas arise within or near the vestibular ganglion. Most vestibular schwannomas are known as hypovascular tumors. Estimated hemorrhage rate of vestibular schwannoma is less than 1%. Therefore, even a large tumor can be totally resected without risk of tumor bleeding or morbidity (1, 2, 5). However, unlike hypovascular vestibular schwannoma, hypervascular vestibular schwannoma is very risky for bleeding because of its rich abnormal tumor vessels. In the case of hypervascular vestibular schwannoma, multi-staged surgical approach is required to reduce morbidity (6, 7, 8).
Yamakami et al. (3) retrospectively reviewed clinical characteristics of 78 patients with unilateral vestibular schwannomas. There were five hypervascular vestibular schwannomas of the 78 tumor patients. Hypervascular vestibular schwannoma was significantly more common at younger age group (29 ± 12 vs. 52 ± 16 years old). The hypervascular vestibular schwannomas appeared to be larger than hypovascular tumors. In addition, hypervascular vestibular schwannomas seemed to have more solid portion with less cystic change (3).
The typical MR imaging (MRI) features of vestibular schwan-noma is heterogeneously enhancing CPA mass without intratumoral and peritumoral signal void structures. However, in hypervascular vestibular schwannomas, multiple flow void structures are seen in and around the mass. Signal void structures on the surface of hypervascular vestibular schwannoma represent large draining veins (2, 3). Notably, Yamakami et al. (3) and Han et al. (4) reported that all hypervascular vestibular schwannomas had multiple flow voids on MRI. Therefore, flow voids on tumor surface on MRI can help radiologists to make diagnosis of hypervascular vestibular schwannoma.
The characteristic angiographical findings of hypervascular vestibular schwannoma are extensive tumor vessels, tumor stains, and early filling of draining veins. As shown in this case, hypervascular vestibular schwannomas are usually supplied by vertebrobasilar arteries (from the posterior inferior cerebellar artery, the anterior inferior cerebellar artery and the superior cerebellar artery), probably related to its location (3, 4, 7). According to Yamakami et al. (3) and Han et al. (4), all hypervascular vestibular schwannomas had vascular supply from posterior circulation. Because of the arteriovenous shunt, large draining veins with early venous filling are also observed.
To date, there has been no radiological report concerning rCBV of hypervascular vestibular schwannoma. Increased rCBV on MR perfusion imaging means rich blood supply of hypervascular tumor or hypermetabolism of malignant tumor. In this case, increased rCBV of hypervascular vestibular schwannoma could be due to the hypervascularity of the tumor.
Han et al. (4) reported the bleeding risk of hypervascular vestibular schwannoma. In that study, all four hypervascular vestibular schwannomas were found having subarachnoid bleeding, with many abnormal vessels distributing on the surface of tumors. Therefore, controlled hypotension anesthesia was needed during total resection of hypervascular vestibular schwannoma.
Our case showed variable imaging features of hypervascular vestibular schwannoma by enhanced CT, MRI, perfusion MRI, and cerebral angiography. It is important to be familiar with multiple characteristic features of hypervascular vestibular schwannoma to make preoperative diagnosis of hypervascular vestibular 200schwannoma to reduce morbidity and mortality from tumor bleeding. Angiography with embolization might be needed to reduce bleeding risk. Neurosurgeons may need multi-staged resection of hypervascular vestibular schwannoma for controlling tumor bleeding (3, 4, 7).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download