Abstract
Scimitar syndrome is a rare, combined abnormality of bronchopulmonary development and pulmonary vascular development characterized by an anomalous pulmonary venous return to the inferior vena cava. Although the scimitar syndrome has been associated with many anomalies, a tracheoesophageal fistula (TEF), especially from H-type, is extremely rare and only a few cases have been reported without detailed descriptions. Herein we report a rare case of scimitar syndrome with H-type TEF and multiple anomalies in a newborn infant, with a special emphasis on the imaging features associated with the radiologic diagnosis using an electrocardiography-gated computed tomography.
A scimitar syndrome (SS) is a rare congenital anomaly with effects most often noted on the right side of the body. This syndrome is characterized by a partial or complete anomalous pulmonary venous return to the inferior vena cava (IVC), which used to be referred to as the "Scimitar vein" (1). SS has been associated with a variety of potential anomalies including hypoplasia of the pulmonary artery, systemic arterial blood supply to the affected lobe from the descending aorta, dextrocardia, pulmonary sequestration, and persistent left superior vena cava (2). In addition to these anomalies, SS can be accompanied by a tracheoesophageal fistula (TEF), however, this is extremely rare. The chest computed tomography (CT) scan is considered to be one of the most accurate, noninvasive diagnostic methods for SS and its various associated anomalies. However, conventional CT scans, especially in infants, are limited by the patient's respiration and cardiac motion. Here, we present a case of a newborn infant with SS and associated H-type TEF diagnosed by using electrocardiography (ECG)-gated chest CT with detailed image descriptions.
A 3300 g male infant was born at 40 weeks of gestation via normal vaginal delivery as the first baby of a 32-year-old mother. No specific fetal anomalies were identified on the prenatal screening tests, including a fetal ultrasound. The newborn had a mild tachypnea and chest wall retraction while breathing, but his general appearance was good and he displayed a normal level of activity. His Apgar scores were 7 at 1 minute and 8 at 5 minutes. With the exception of mild distention of the abdomen, no external anomalies were detected during a physical examination. The initial chest radiograph showed a nearly complete opacification of the right hemithorax with shifting of the mediastinum to the right side (Fig. 1A). The chest radiograph also revealed a segmented T10 vertebral body, a left 9-10th rib fusion and a diffuse air-filled intestine. The nasogastric tube was successfully inserted without knotting and the initial vital signs showed a mild oxygen desaturation (pulse-oximetry, SaO2 92%). Supplemental oxygen was started by nasal cannula at 2 liters per minute. During his hospitalization, the newborn showed a persistent mild desaturation and recurrent tachypnea despite the continuous oxygen supply. His oral secretion and abdominal distension also gradually increased. Our physicians suspected those conditions were related to an airway aspiration regardless of lung anomalies.
An echocardiography was performed as part of an initial work-up of anomalies and demonstrated a hypoplastic right pulmonary artery, coarctation of the aorta and a small atrial septal defect. The further evaluation included an ECG-gated volume chest CT examination using a 320-row detector CT scanner (Aquilion One, Toshiba Medical Systems, Otawara, Japan). The detector width was set at 8 cm to cover the entire thorax. As the newborn's heart rate was 133-150 beats per minute, the CT images were obtained by using a prospectively triggered scan with a systolic data acquisition at 40% of RR interval. The scan parameters were as follows: 350-ms gantry rotation time, 100 kVp tube voltages, 70 mA tube current. Nonionic contrast material (350 mgI/mL, 7 mL) was administered at 0.7 mL/s followed by a 30 mL saline flush. The total dose length product was 21.3 mGy*cm [0.3 mSv, k = 0.014 mSv/(mGy*cm)]. Axial images of the whole thorax scan range were reconstructed from 40% of the RR interval at a slice thickness of 1 mm. The thin slice (0.5 mm) volume data were sent to the Aquarius Workstation (Terarecon, San Mateo, CA, USA) for multiplanar display and three-dimensional (3D) reconstruction.
The axial CT scan showed a relatively smaller volume of the right lung compared to the left lung and a deviated mediastinum to the right side. The right bronchus appeared as a single main bronchus without branching. The right main pulmonary artery showed a very small diameter (2 mm) and there was no evidence of right pulmonary venous structures that provided for drainage to the left atrium. An abnormal systemic arterial supply was seen on the hypoplastic right lung from the abdominal aorta. An anomalous venous drainage into the IVC was detected below the level of the right hemidiaphragm and showed the appearance of a Turkish "Scimitar" sword. Radiologically, these characteristic CT findings were sufficient to confirm the diagnosis of SS (Fig. 1B-E). Other associated abnormal CT findings were also observed by echocardiography and chest radiograph, such as focal aortic stenosis at the aortic isthmus suggesting coarctation of aorta, non-fused T10 vertebra (hemivertebra) and fused left 9th to 10th ribs at the vertebral attachment site.
This infant was also clinically suspected of airway aspiration. Therefore, we carefully evaluated his CT images focused on the airways and esophagus. On the axial and 3D volume rendered CT images, a focal nodular out-pouching of the posterior tracheal wall was noted at 1 cm above the level of the carina (Fig. 1F, G). Correlating with the clinically suspicious recurrent aspiration and a gradually increased abdominal distension, we postulated that this focal indentation was a small tract between the trachea and esophagus suggestive of TEF. A barium esophagography showed a short fistula tract between the esophagus and trachea without esophageal atresia and hereby confirmed the diagnosis of H-type TEF (Fig. 1H).
The SS is a rare and complex partial abnormality of the pulmonary venous return due to a connection failure between the right pulmonary veins and the left atrium during the fetal development. The estimated incidence of SS is 1-3 per 100000 live births; however, this incidence may be underestimated because many patients are asymptomatic. This syndrome almost always manifests exclusively as a right-sided anomaly. A right pulmonary hypoplasia subsequently results in a rightward deviation of the heart with elevation of the right hemidiaphragm, hypoplastic right pulmonary artery and vascular supply of the right lower lobe from the abdominal aorta or its main branches. Many variants have been reported and the incidence of associated congenital cardiovascular abnormalities include atrial septum defects, ventricular septum defects, coarctation of the aorta, abnormalities of the aortic arch and abnormal relationships of the pulmonary artery and bronchi seen in 36% of children and 75% of neonates (3). Other commonly associated anomalies are vertebral anomalies (e.g., hemivertebra), scoliosis and genitourinary anomalies (4).
The etiology of SS remains unclear, but is thought to originate from a basic abnormality in the early pulmonary embryogenesis. During the course of a normal lung development, its primary blood supply changes from a plexus derived from the post-branchial descending aorta to the portion of the 6th aortic arch that becomes the pulmonary artery, a transition that is complete after the 7th week of gestation (5). Insults in this course can lead to the persistence of a systemic arterial supply to the right lung from the abdominal aorta and the underdevelopment of the right pulmonary artery and right lung.
TEF is the most common of the congenital anomalies that often accompany esophageal atresia (EA). Of the five subtypes described originally, TEF without EA (or H-type TEF) is an extremely rare form of isolated TEF in infants which accounts for approximately 4% of all congenital tracheoesophageal malformations. The trachea, esophagus and lungs are divided from the foregut during the 4th week of embryonic life into a ventral respiratory part and a dorsal esophageal part. A longitudinal tracheoesophageal fold in the ventral respiratory diverticulum that fuses to form the tracheoesophageal septum separates the laryngotracheal tube from the esophagus. If the tracheoesophageal septum is deviated posteriorly, the separation of the esophagus from the laryngotracheal tube is incomplete and results in a concurrent esophageal atresia or TEF (6). The pathologic mechanism leading to TEF is unknown, but is thought to be a multifactorial disease involving both genetic and environmental factors such as VACTERL association (vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, and limb abnormalities), and maternal exposure to infections, drugs, diabetes, etc. (7).
The etiological relationship between SS and TEF remains unclear. SS with TEF is extremely rare and only two cases have been reported until yet. The first case was a case of SS with TEF as VACTERL association without a detailed description of imaging findings (2). The second case involved a SS with H-type TEF similar to the patient in the present case (8).
The diagnosis of SS is based on its clinical presentation and various imaging studies including transthoracic or transesophageal echocardiography, angiography, CT, and magnetic resonance angiography. An echocardiography can be used as a first step imaging study in symptomatic patients with abnormal cardiac physical examination results. If the characteristic curved opacity of the scimitar vein is present on the conventional chest radiograph ("Scimitar Sign"), the diagnosis can also be made with confidence. However, the Scimitar vein can be masked by the overlying cardiac shadow, especially in infants and young children. Therefore, other imaging modalities are required for accurate diagnosis. The chest CT scan was considered one of the most accurate, noninvasive diagnostic methods for SS (9). However, a conventional CT is limited by the patient's respiration and cardiac motion and this is especially true for infants due to their inability to breath-hold and a normal heart rate over 100 beats per minutes. Recent advances in the CT technology such as the EGC-gated cardiac CT (320-detector CT volume scanner), have allowed for a more rapid imaging of infants with congenital heart disease by providing better spatial and temporal resolution, fewer motion artifacts, a shorter scan time, a decreased need for sedation and the requirement of less contrast media. It also has the advantage of reducing the patient radiation exposure by reducing the overlapping helical rotation and a minimal penumbral overbeaming with volumetric scanning (10). Additionally, the currently available various multiplanar reformation techniques provides for a more sophisticated image and an excellent visualization of the vascular and tracheobronchial anatomy. Beyond its advantages pertaining to the diagnosis, the EGC-gated cardiac CT allows for the systematic evaluation required in order to detect other abnormalities often associated with SS.
In the present case, the initial chest radiograph and echocardiography results were not sufficient to implicate the Scimitar vein. A contrast-enhanced ECG-gated volume chest CT scan was performed for diagnosis. We obtained a high resolution image with minimal radiation exposure and the lowest possible amount of injected contrast agent. We confirmed this infant as having a complicated SS with various associated anomalies, such as coarctation of the aorta, hemivertebra, rib fusion, and H-type TEF. During the diagnostic process, the 3D reconstruction image played an important role regarding the suspicion of TEF by showing a small tract-like indentation at the posterior side of the trachea. Although H-type TEF diagnosed by 3D reconstruction imaging had not been reported previously, this modality proved to be valuable for investigating a suspected SS in a complicated case.
Figures and Tables
Fig. 1
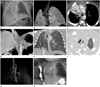
A newborn male infant shows tachypnea and chest wall retraction while breathing.
A. A chest radiograph demonstrates an almost completely opacified right hemithorax with a mediastinal shift to the right side. Additionally, hemivertebra of T10 (black arrow), left 9-10th rib fusion (arrowhead), and a gaseous distended stomach and intestine are also seen. The tip of the nasogastric tube was seen within the stomach (nasogastric tube; white arrows).
B. A minimal intensity projection image showing the hypoplastic right lung with a single right lobar bronchus.
C. An axial electrocardiography (ECG)-gated volume chest CT showing a hypoplastic right pulmonary artery measuring 2.27 mm in diameter (main pulmonary artery: 9.5 mm, left pulmonary artery: 4.94 mm).
D. A coronal reformatted ECG-gated volume chest CT showing an aberrant systemic artery to the hypoplastic right lung from the abdominal aorta (arrow).
E. A coronal reformatted ECG-gated volume chest CT showing the curved anomalous pulmonary veins (scimitar vein, arrow) descending vertically, draining the right lung, and entering into the inferior vena cava.
F. An axial ECG-gated volume chest CT showing the cardiac dextroposition and the focal out-pouching wall at the tracheal posterior wall (arrow).
G. A three-dimensional tracheobronchial tree image demonstrates the focal retracted posterior wall of the trachea, 1 cm above the level of the carina (arrow).
H. Barium esophagography revealed a slit like fistula tract between the trachea and the esophagus (arrow). A final spot view visualized the barium-coated trachea and single right bronchus. The distal esophagus is intact and shows normal passage of contrast media into the stomach, suggesting an H-type tracheoesophageal fistula.
Note.-A = abdominal aorta, AA = ascending aorta, C = celiac trunk, DA = descending aorta, E = esophagus, IVC = inferior vena cava, LLB = left lower lobar bronchus, LMB = left main bronchus, LUB = left upper lobar bronchus, LUL = left upper lung, MPA = main pulmonary artery, RB = right lobar bronchus, RPA = right pulmonary artery, SVC = superior vena cava, T = trachea
References
1. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage "scimitar syndrome". Bull Johns Hopkins Hosp. 1960; 107:1–21.
2. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years' experience and results of repair. J Thorac Cardiovasc Surg. 1996; 112:1161–1168. discussion 1168-1169.
3. Khalilzadeh S, Hassanzad M, Khodayari AA. Scimitar syndrome. Arch Iran Med. 2009; 12:79–81.
4. Ahamed MF, Al Hameed F. Hypogenetic lung syndrome in an adolescent: imaging findings with short review. Ann Thorac Med. 2008; 3:60–63.
5. Huddleston CB, Mendeloff EN. Scimitar syndrome. Adv Card Surg. 1999; 11:161–178.
6. Clark DC. Esophageal atresia and tracheoesophageal fistula. Am Fam Physician. 1999; 59:910–916. 919–920.
7. de Jong EM, Felix JF, de Klein A, Tibboel D. Etiology of esophageal atresia and tracheoesophageal fistula: "mind the gap". Curr Gastroenterol Rep. 2010; 12:215–222.
8. Lastinger A, El Yaman M, Gustafson R, Yossuck P. Scimitar Syndrome and H-type Tracheo-esophageal Fistula in a Newborn Infant. Pediatr Neonatol. 2013; pii: S1875-9572(13)00154-X.
9. Legras A, Guinet C, Alifano M, Lepilliez A, Régnard JF. A case of variant scimitar syndrome. Chest. 2012; 142:1039–1041.
10. Podberesky DJ, Angel E, Yoshizumi TT, Toncheva G, Salisbury SR, Alsip C, et al. Radiation dose estimation for prospective and retrospective ECG-gated cardiac CT angiography in infants and small children using a 320-MDCT volume scanner. AJR Am J Roentgenol. 2012; 199:1129–1135.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download