Abstract
Anaplastic large cell lymphoma (ALCL) is a rare T cell lymphoma composed of CD30-positive lymphoid cells. Most ALCLs present as nodal disease, with skin, bone, soft tissue, lung, and liver as common extranodal sites. ALCL rarely occurs in the central nervous system and is even more infrequent in the dura of the brain. We report a case of dural-based ALCL secondary to systemic disease in a 17-year-old male that mimicked meningioma on magnetic resonance imaging and angiography.
Meningeal involvement by malignant lymphomas most commonly represents secondary spread from advanced systemic disease. Usually manifesting as a diffuse leptomeningeal infiltrates, it is reportedly detected in 4-10% of patients with systemic lymphomas and, in the majority of cases, is of the aggressive histologic type (1, 2). Lymphoma rarely presents as a localized mass within the dura. When it does present in this fashion it is frequently mistaken for meningioma. The majority of cases of dural lymphomas reported in the literature have been primary mucosa-associated lymphoid tissue (MALT) type lymphomas (1, 2). Dural-based anaplastic large cell lymphoma (ALCL), which is primary or secondary, is an extremely rare entity. Few cases have been reported.
In this report, we present computed tomography (CT), magnetic resonance imaging (MRI), and angiographic findings and histopathologic features of a case of dural ALCL in which the initial manifestation of disease was a localized dural mass. Subsequent investigations confirmed the presence of systemic involvement of the disease.
A 17-year-old immunocompetent male presented with a month-long history of persistent headache of increasing severity. He noted episodic nausea and vomiting in association with the headache that was exacerbated by exertion. Familial history was not remarkable. There were no specific findings on physical examination including neurologic evaluation, and routine laboratory tests were normal. Precontrast axial CT revealed a homogenous broad dural-based semi-ovoid-shaped extra-axial mass in the left temporal lobe (Fig. 1A). T1-weighted and T2-weighted MRI (Fig. 1B, C, respectively) showed a slightly heterogeneous broad dural-based extra-axial mass in the left temporal lobe. The signal intensity of the lesion was similar to that of parenchymal gray matter. Gadolinium-enhanced coronal MRI (Fig. 1D) revealed intense and homogenous enhancement with a dural-tail sign. Cerebral angiography was performed thereafter, and left external carotid artery angiogram revealed a sunburst appearance of vessels supplied by the middle meningeal artery in the arterial phase (Fig. 1E) and prolonged vascular stain in the late venous phase (Fig. 1F). On the basis of MRI and angiographic findings, left temporal convexity meningioma was diagnosed. The patient underwent left frontotemporoparietal craniotomy and the tumor was resected.
Histopathologic examination under high power revealed a population of large neoplastic cells (Fig. 2A). Immunohistochemical analysis demonstrated that the tumor cells were diffusely and strongly positive for a pan-T cell marker (CD3), negative for pan-B cell marker (CD20), and positive for CD30 (Fig. 2B) and anaplastic lymphoma kinase protein. These results supported a diagnosis of ALCL.
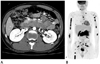
The patient had anemia postoperatively. Gastroscopy and abdominopelvic CT were performed to rule out gastrointestinal bleeding as the cause of anemia. On gastroscopy, a 2.5 cm mass with central deep ulceration was noted in the distal second portion of the duodenum. Two dot-like black spots were seen at the central portion of the ulcer (image not shown) and biopsy was performed. Pathologic examination showed that the tissue was histologically similar to the brain lesion. Abdominopelvic CT showed multiple enlarged and conglomerated mesenteric lymph nodes, suggesting mesenteric lymphoma (Fig. 3A). Positron emission tomography imaging demonstrated multiple areas of hot uptake, such as the mesenteric and pelvic lymph nodes and bones of the upper and lower extremities, suggesting systemic ALCL (Fig. 3B).
The patient was transferred to another hospital for bone marrow transplantation.
Central nervous system (CNS) involvement of non-Hodgkin's lymphoma falls into one of three categories (3): primary CNS lymphomas, disseminated lymphomas with CNS involvement, and primary dural lymphomas.
Secondary CNS involvement is seen in 5-9% of all non-Hodgkin lymphomas, usually in the form of diffuse leptomeningeal infiltrates or intraparenchymal masses (4). Skull and dural involvement is much more frequent with this type of lymphoma than in primary CNS lymphomas. Invasion restricted to the intracranial dura is rare (5). The main histologic subtypes of lymphomas that commonly involve the CNS are all high-grade malignancies, including diffuse large B cell lymphoma, Burkitt's lymphoma, lymphoblastic lymphoma, and, less commonly, peripheral T cell lymphoma not otherwise specified (6).
Primary lymphomas occurring in the meninges are rare. In the leptomeninges, these are typically high-grade B cell lymphomas, although occasional cases of primary T cell lymphomas have also been described (7). On the other hand, primary lymphomas in the dura are predominantly of a low-grade B cell type (6).
To the best of our knowledge, dural-based lymphomas in the literature that mimic meningioma due to their presentation as a solitary mass almost always occur with primary lymphoma rather than secondary lymphoma. MALT lymphomas represent the largest group of primary dural lymphomas, with around 30 cases having been described in the literature (6). MALT lymphoma is usually indolent and rarely becomes disseminated. Thus, dural MALT lymphoma is almost always localized without systemic involvement (3).
Clinically, tumors with dural-based metastasis that mimic meningioma are very rare and mostly described in case reports from diverse primary locations. Historically, the most common primary sites in surgically resected dural metastases have been breast, prostate, and kidney (8). Secondary dural lymphoma that mimics meningioma is an extremely rare phenomenon.
In conclusion, lymphoma should be considered in the differential diagnosis of meningioma, although it appears to be a considerably rare occurrence. In addition, dural-based lymphoma is possible and can be not only the primary low-grade B cell type, but a high grade type such as ALCL, which may arise from systemic disease.
Figures and Tables
Fig. 1
Radiologic findings of dural anaplastic large cell lymphoma in a 17-year-old man.
A. Precontrast axial CT shows homogenous broad dural-based semi-ovoid extra-axial mass in the left temporal lobe.
B, C. On T1-weighted (B) and T2-weighted (C) MR images, a heterogeneous broad dural-based extra-axial mass in the left temporal lobe has similar signal intensity as parenchymal gray matter.
D. Gadolinium-enhanced coronal T1-weighted MR images show intense and homogenous enhancement of the mass with dural-tail sign.
E, F. Left external carotid artery angiogram demonstrates a prominent core vascular supply with a sunburst appearance supplied by the middle meningeal artery in the arterial phase (E) and prolonged vascular stain in the late venous phase (F).
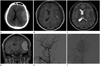
Fig. 2
Microscopic findings of dural anaplastic large cell lymphoma in a 17-year-old man.
A. Histopathologic examination under high power shows a diffuse population of large, atypical lymphoid cells with abundant cytoplasm; round and pleomorphic nuclei; and prominent nucleoli (H&E, × 200).
B. Immunohistological examination under high power shows that tumor cells are immunoreactive for CD30 (immunoperoxidase, × 400).

Fig. 3
Systemic involvement of dural anaplastic large cell lymphoma in a 17-year-old man.
A. Axial portal phase CT image reveals multiple enlarged and conglomerated mesenteric lymph nodes (arrows).
B. Positron emission tomography image reveals multiple areas of hot uptake such as mesenteric and pelvic lymph nodes and bones of the upper and lower extremities.
References
1. Griffin JW, Thompson RW, Mitchinson MJ, De Kiewiet JC, Welland FH. Lymphomatous leptomeningitis. Am J Med. 1971; 51:200–208.
2. Amaker BH, Ghatak NR, Jebraili SA, Ferreira-Gonzalez A, Kornstein MJ. Primary T-cell-rich B-cell lymphoma masquerading as a meningioma. Arch Pathol Lab Med. 2000; 124:1700–1703.
3. Matmati K, Matmati N, Hannun YA, Rumboldt Z, Patel S, Lazarchick J, et al. Dural MALT lymphoma with disseminated disease. Hematol Rep. 2010; 2:e10.
4. Lantos PL, Louis DN, Rosenblum MK, Kleihues P. Tumours of the nervous system. In : Graham DI, Lantos PL, editors. Greenfield's Neuropathology. 7th ed. London: Arnold;2002. vol. 2:p. 950–959.
5. Miller DC, Hochberg FH, Harris NL, Gruber ML, Louis DN, Cohen H. Pathology with clinical correlations of primary central nervous system non-Hodgkin's lymphoma. The Massachusetts General Hospital experience 1958-1989. Cancer. 1994; 74:1383–1397.
6. Low I, Allen J. Low-grade follicular lymphoma in the dura: rare mimic of meningioma. Neuropathology. 2006; 26:564–568.
7. Villegas E, Villà S, López-Guillermo A, Petit J, Ribalta T, Graus F. Primary central nervous system lymphoma of T-cell origin: description of two cases and review of the literature. J Neurooncol. 1997; 34:157–161.
8. Savage NM, Alleyne CH, Vender JR, Figueroa R, Zhang H, Samuel TA, et al. Dural-based metastatic carcinomas mimicking primary CNS neoplasia: report of 7 cases emphasizing the role of timely surgery and accurate pathologic evaluation. Int J Clin Exp Pathol. 2011; 4:530–540.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download