Abstract
The pineal gland is an unusual site for brain metastasis, and a solitary pineal gland metastasis is rare. A 71-year-old man presented with dizziness, gait disturbance, and memory impairment. Brain computed tomography revealed a solitary hyperdense mass with central calcification in the pineal region and obstructive hydrocephalus. Brain magnetic resonance images showed a heterogeneously enhancing mass with intratumoral calcification and microcysts. Metastatic squamous cell carcinoma was diagnosed following an endoscopic biopsy. A systemic review revealed that the primary site of the carcinoma was the lung. Although rare, metastasis should be considered in the differential diagnosis of pineal region tumors, especially in elderly patients with a pineal tumor that presents unusual imaging findings.
The brain is a common site of metastasis from lung cancer; however, the pineal region is an unusual site for metastasis, and a solitary pineal gland metastasis is much rarer (1, 2, 3, 4, 5, 6). The prevalence of metastasis to the pineal region accounts for 0.4-3.8% of brain metastasis from solid tumors (1). The most common primary tumor of the pineal metastasis is the lung, and small cell carcinoma is the most frequent histological type of lung cancer (2). Most patients with pineal metastasis are asymptomatic, and are usually diagnosed at autopsy (2, 3, 4, 5).
Our patient presented with a pineal mass and obstructive hydrocephalus, and was diagnosed with pineal metastasis from squamous cell carcinoma of the lung. A pineal metastasis as the initial presentation of the lung cancer is a rare clinical manifestation, and this histological type seems rare.
A 71-year-old man presented with a 1-month history of dizziness, gait disturbance, and memory impairment. These symptoms worsened progressively.
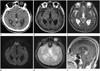
He underwent brain computed tomography (CT) and magnetic resonance (MR) imaging. The brain CT scan revealed a solitary well-defined hyperdense (50 Hounsfield unit) mass with central calcification in the pineal region and obstructive hydrocephalus. Brain MR images showed a 2.3 × 1.6 × 2.0 cm well-defined lobulated mass in the pineal area and obstructive hydrocephalus due to compression of the cerebral aqueduct. The signal intensities of the mass were low on T1 weighted images (WI) and isointense on T2WI. No restricted diffusion was observed on either diffusion-weighted imaging or an apparent diffusion coefficient map. It contained a central dark signal intensity focus due to the engulfment of pineal calcification on a gradient echo image, and T2 high signal intensity foci of microcysts. The mass enhanced heterogeneously after the administration of contrast media (Fig. 1).
Based on the imaging findings of the pineal mass, we considered pineal parenchymal tumor of intermediate differentiation (PPTID) (World Health Organization grade II or III) or a germinoma as possible differential diagnoses. PPTIDs can appear at any age. PPTIDs show high signal intensity on T2WI, and may demonstrate cystic areas. Approximately 90% of patients with germinomas are younger than 20 years old. The germinoma may demonstrate a mass with engulfed pineal calcification, and show either isointense or high signal intensity on T1WI and T2WI, and restricted diffusion may be seen because of its high cellularity (1). Thus, we considered that a PPTID was a more plausible diagnosis given his age.
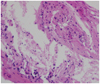
Serum levels of tumor markers, such as CEA, AFP, PSA, SCC, CA-125, and CA-153 were at normal levels. For a confirmatory diagnosis of the tumor and management of hydrocephalus, he underwent an endoscopic third ventriculostomy with a biopsy. The mass was then diagnosed pathologically as a metastatic squamous cell carcinoma. The histopathological examination showed atypical stratified squamous cells with keratin (Fig. 2).
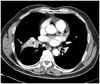
Then, a subsequent systemic review for the primary lesion was performed. A plain chest radiograph and chest CT revealed a central mass in the right middle lobe of the lung and associated atelectasis (Fig. 3). A bronchoscopic lung biopsy confirmed squamous cell carcinoma.
Although chemotherapy for the lung cancer was delayed because of his poor general condition, a Gamma Knife surgery was performed for the pineal gland metastasis. About 3 months after the Gamma Knife surgery, all initial symptoms had improved with mild residual cognitive impairment. The follow-up brain MR imaging showed a decrease in the size of the pineal mass (1.1 × 0.8 × 0.6 cm) and the improvement of hydrocephalus.
A solitary pineal gland metastasis in the brain is rarely reported. In addition, it is unusual to present with symptoms due to an isolated metastatic pineal tumor before the diagnosis of the primary cancer (1, 2, 3, 4, 5, 6).
Foster described the first report of pineal gland metastasis in 1858 (4). According to previous literature, the most common primary tumors are carcinomas of the lung, breast, stomach, esophagus, rectum, and kidney. There are also some case reports of pineal region metastases from hematological malignancies and melanomas. The most common primary tumor is lung cancer, and the most frequent histological type of lung cancer is small cell carcinoma (2, 4). Our patient was confirmed to have squamous cell carcinoma of the lung; this histological type seems infrequent. Previous autopsy reports indicate a prevalence of 0.4-3.8% in patients with solid tumors (1). In the past, metastases to the pineal gland were found at autopsy, but recently the availability of imaging modalities, such as MRI, has helped with earlier detection (5).
The mechanism of pineal metastasis is only partially understood. Ortega et al. (7) explained the mechanism as hematogenous spread through the posterior choroidal arteries (2, 5). Because of the lack of blood-brain barrier, the pineal gland is more vulnerable to such hematogenous spread. Kashiwagi et al. (8) suggested that the numerous sinusoidal vessels with no perivascular glial sheets in the pineal gland increase the vascular permeability, contributing to hematogenous metastasis (2).
When encountering a solitary pineal mass, any history of malignancy and the age of the patient are the important factors in differentiating the lesion. About 90% of supratentorial lesions represent metastases in patients with a known history of malignancy. Most patients with primary pineal tumors are younger than 30 years old (2, 4, 5, 6, 8).
Because the pineal gland is located adjacent to the opening of the cerebral aqueduct, pineal metastasis can compress the cerebral aqueduct and result in obstructive hydrocephalus. There are several treatment options for obstructive hydrocephalus due to pineal metastasis, including external ventricular drainage, ventriculoperitoneal shunting, and neuroendoscopic third ventriculostomy. External ventricular drainage and ventriculoperitoneal shunting can be associated with a risk of infection and peritoneal seeding (2). In this case, we obtained histological specimens from the pineal tumor by an endoscopic brain biopsy to confirm the histological diagnosis, and we simultaneously performed a third ventriculostomy to relieve the hydrocephalus and improve the patient's general condition. This third ventriculostomy could reduce the risk of infection and peritoneal neoplastic dissemination, compared with the other treatment options. The Gamma Knife surgery was also effective in the treatment of the tumor.
Our patient was a 71-year-old man and presented with dizziness, gait disturbance, and memory impairment. Brain CT and MR imaging revealed a solitary pineal mass with obstructive hydrocephalus. His symptoms were considered to be related to the clinical manifestations of hydrocephalus. We considered that the pineal mass may be a PPTID. However, PPTIDs usually show high signal intensity on T2WI. The imaging findings of the pineal tumor were not compatible with any specific pineal neoplasm. An endoscopic biopsy of the pineal tumor revealed a metastatic squamous cell carcinoma. A subsequent systemic review was performed, and the primary site of the carcinoma was found to be the lung.
In conclusion, metastasis should be considered in the differential diagnosis of a pineal region tumor, especially in elderly patients, patients with a known history of malignancy, or patients with a pineal tumor that presents unusual imaging findings.
Figures and Tables
Fig. 1
A non-contrast-enhanced axial CT image shows a solitary well-defined hyperdense (50 Hounsfield units) mass (arrow, A) with central calcification in the pineal region. A well-defined lobulated mass in the pineal area shows intemediate signal intensity on axial T1 weighted images (WI) (B) and isosignal intensity on T2WI (C). There is no restricted diffusion on diffusion-weighted imaging (D). The mass contains a central dark signal intensity focus due to the engulfment of pineal calcification on gradient echo image (arrow, E), and T2 high signal intensity foci of microcysts (arrow, C). Contrast-enhanced sagittal T1WI (F) shows heterogeneous enhancement of the tumor. The pineal mass results in obstructive hydrocephalus (asterisk, F).
References
1. Smith AB, Rushing EJ, Smirniotopoulos JG. From the archives of the AFIP: lesions of the pineal region: radiologic-pathologic correlation. Radiographics. 2010; 30:2001–2020.
2. Nemoto K, Aoshiba K, Itoh M, Semba S, Tsuji T, Adachi H, et al. Isolated pineal region metastasis from lung adenocarcinoma with obstructive hydrocephalus: a case report. J Med Case Rep. 2013; 7:71.
3. Vaquero J, Martínez R, Magallón R, Ramiro J. Intracranial metastases to the pineal region. Report of three cases. J Neurosurg Sci. 1991; 35:55–55.
4. Oztekin O, Savas R, Ozan E, Apaydin M, Yasar O, Adibelli ZH, et al. Pineal gland metastasis of auricular squamous cell carcinoma: an unusual case and literature review. Radiol Oncol. 2009; 43:175–179.
5. Samanci Y, Iplikcioglu C, Ozek E, Ozcan D, Marangozoglu B. Lung carcinoma metastasis presenting as a pineal region tumor. Neurocirugia (Astur). 2011; 22:579–582.
6. Ahn JY, Chung YS, Kwon SO, Huh R, Chung SS. Isolated pineal region metastasis of small cell lung cancer. J Clin Neurosci. 2005; 12:691–693.
7. Ortega P, Malamud N, Shimkin MB. Metastasis to the pineal body. AMA Arch Pathol. 1951; 52:518–528.
8. Kashiwagi S, Hatano M, Yokoyama T. Metastatic small cell carcinoma to the pineal body: case report. Neurosurgery. 1989; 25:810–813.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download