Abstract
A 76-year-old man with fatigue was admitted to our hospital. Computed tomography (CT) and magnetic resonance imaging (MRI) showed a well-circumscribed mass with delayed heterogeneous enhancement between the pancreas head and the second portion of the duodenum. A surgical resection was performed and a lymphoepithelioma-like carcinoma (LEC) was pathologically confirmed, originating from the head of the pancreas. After the surgery, the patient did not have additional treatment and his LEC did not recur for four years and 10 months. We report MRI and CT findings with histopathologic findings of an extremely rare case of LEC arising from the pancreatic head.
An undifferentiated carcinoma with massive lymphocytic stromal infiltration is defined as a lymphoepithelioma-like carcinoma (LEC) (1). Although LEC often develops in the nasopharynx, it has been rarely reported in other organs and some LECs are associated with Epstein-Barr virus (EBV) infection (2). To the best of our knowledge, a few cases of pancreatic LEC have been reported recently and no magnetic resonance imaging (MRI) of LEC arising from the pancreatic head has been reported yet (2, 3). In the MRI of our patient with pancreatic LEC, the LEC was presented as an oval, expansive and well-demarcated mass with heterogeneous rim and septal enhancement in the delayed phase. These imaging findings were well-correlated with the histological finding of multiple lympho-epitheloid follicles surrounded by fibrous tissue.
A 76-year-old man with fatigue and abdominal discomfort was admitted to the outpatient department of internal medicine at our hospital. The laboratory blood analysis showed a serum level of carbohydrate antigen (CA) 19-9 of 74.24 u/mL with a normal range between 0 and 37 u/mL. However, the levels of other markers were within normal range.
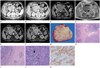
A dynamic abdominal computed tomography (CT) was performed to detect the cause of abdominal discomfort. The arterial phase CT revealed a well-defined, relatively homogeneous enhancement of the mass in the pancreas head abutting to the second portion of the duodenum (Fig. 1A). The growth pattern of the mass was expansive and the adjacent vascular structure was displaced rather than encased (Fig. 1A). The pancreatic duct of the head portion was mildly dilated without proximal duct dilatation. The mass appeared as more heterogeneous enhancement in the delayed phase of abdominal CT (Fig. 1B).
MRI and MR cholangio-pancreatography (MRCP) were performed with a 1.5T magnet (Intera, Philips Medical Systems, The Netherlands). It included axial and coronal T1-weighted gradient echo with fat suppression; T2-weighted axial and coronal single-turbo spin echo with fat suppression; two-dimensional and three-dimensional MRCP and three-dimensional T1-weighted gradient echo before and after administration of gadolinium contrast agent. Scanning times of contrast-enhanced sequences that were used in the arterial, venous and delayed phases were 20 and 50 seconds and 5 minutes, respectively. The MRI showed a mass with intermediate to low signal intensity on T1-weighted images (Fig. 1C) and intermediate to high signal intensity on T2-weighted images (Fig. 1D). On the early phase of the contrast-enhanced T1-weighted images, the mass was present with a relatively homogeneous enhancement (Fig. 1E), but the mass had a reticular or "spider web" appearance characterized by poorly enhanced nodular portions and surrounding brightly enhanced rim and septal portions, similar to those in the capsule and septa (Fig. 1F).
We assumed that the mass originated from the duodenum and a gastrointestinal stromal tumor (GIST) was highly suggestive although the serum CA19-9 level was increased. Contrary to our assumption, the surgeon found the mass attached to the upper border of the pancreatic head. Although intra-operative frozen section biopsy proved a carcinoma, surgical wedge resection of the pancreas head was performed because the mass was well capsulated and did not invade adjacent organs including the duodenum, portal vein and the common bile duct.
Grossly, the resected mass measured 6.2 × 4.5 × 3.9 cm in size. The mass showed a well-circumscribed, lobulated gray-white cut surface (Fig. 1G). The microscopic examination showed a well circumscribed tumor margin surrounded by fibrous tissue (Fig. 1H). In the periphery of the tumor, were atrophied pancreatic islets seen (Fig. 1I) proving that the mass originated from the pancreas. Nests were present of undifferentiated or poorly differentiated epitheloid tumor cells and dense lymphoplasmacytic infiltrates. The tumor cells were arranged with a syncytial, trabecular or tubular pattern or with single cells co-existing with lymphoplasmacytes in the photomicrography (Fig. 1J). The immunohistochemical staining showed that the tumor cells were positive for cytokeratin (CK) 19 (Fig. 1K), CK20 and carcinoembryonic antigen and negative for CK7 and thyroid transcription factor-1. Another significant finding was the massive lymphocytic infiltration of the stroma of neoplasm. The infiltrating lymphocytes were immunohistochemically stained with a leukocyte common antigen.
The in situ hybridization for EBV-encoded RNA was negative in the tumor cells and lymphocytes. The mixture of epitheloid tumor cells and extensive lymphocytic infiltrates seemed to suggest the diagnosis of LEC of the pancreas head.
After surgery, the patient did not have additional treatment such as chemotherapy or radiotherapy. The patient had a good prognosis and LEC did not recur for four years and 10 months.
A carcinoma that has undifferentiated features with diffuse stromal lymphoplasmacytic infiltration is defined as a LEC and some LECs are associated with the EBV (1). LEC develops as an undifferentiated variant of non-keratinizing nasopharyngeal carcinoma and is rare in other organs (2). In the previous literature it has been reported as occurring in the hepatobiliary tract (1), stomach (3), colon (4), breast (5), bladder (6), ureter (7), and pancreas (2, 8). LEC very rarely occurs in the pancreas, and Wilentz et al. (9) reported the finding of only one case of lymphoepithelioma-like features that contained EBV-encoded RNA in 13 reported medullary carcinomas of the pancreas.
The CT findings on the previously reported LEC of the pancreas showed well-demarcated, oval, low-density masses with expansile growth patterns rather than infiltrative patterns (8). The dynamic abdominal CT of our patient showed a well-circumscribed mass with heterogeneous enhancement that displacing adjacent structures. Although a mild dilatation of the proximal main pancreatic duct of the pancreatic head and an uncinate process was seen, the distal main pancreatic duct was not dilated. These CT findings of our case were consistent with previously reported radiological findings of pancreatic LEC and differed very significantly from CT findings of an adenocarcinoma of the pancreas, which generally appears as an ill-defined, poorly enhanced soft tissue mass with proximal duct dilatation.
To the best of our knowledge, no literature has reported on MRI findings of a LEC of the pancreas. In our case, the mass in the pancreas head was abutting to the second portion of the duodenum. Therefore, pancreatic MRI and MRCP were performed to better characterize the mass and define its origin. It appeared to have an intermediate to low signal intensity on T1-weighted MR images and an intermediate to high signal intensity on T2-weighted MR images. In the arterial phase of the contrast-enhanced T1-weighted MR images, the mass showed relative homogeneity and good enhancement in the early arterial phase, but in the delayed phase, it appeared as a heterogeneous, reticular and spiderweb-like mass characterized by a bright capsular and septal portion with more poorly enhanced portions. The differential diagnosis of LEC includes GIST, pancreatic neuroendocrine tumor, solitary fibrous tumor and retroperitoneal mesenchymal tumor. But the image findings of LEC are non-specific which makes it difficult to differentiate LEC and previously referred tumors.
Pathologically were multiple fibrous septa with multiple lymphoid and epitheloid follicles seen in the biopsy specimen. We suggested that more highly enhanced areas in the delayed phase might be associated with these fibrous septa and that poorly enhanced portions might be associated with a lymphoepithelioma component. We assumed that radiological and pathological findings were well-correlated.
Based on other literature (2, 8), it was assumed that the prognosis of pancreatic LEC after mass excision might be good. Therefore was an additional treatment unnecessary. There was no reported case of recurrence or metastasis of pancreatic LEC. The presented patient also had a good prognosis without recurrence.
In conclusion, the prognosis of pancreatic LEC is so good that it is important to differentiate it from other forms of pancreatic tumor. Imaging tools may significantly help to diagnose a pancreatic LEC. CT is a helpful imaging technique for the primary detection of pancreatic LEC and the superior contrast resolution of MRI may help to detect and characterize it better. We suggest that pancreatic LEC may be present as an expansile growing, well-demarcated and heterogeneous rim- and septal enhanced mass in the delayed phase of a dynamic MRI. Those radiological findings may be well correlated with histological findings characterized by lymphoid and epitheloid nests surrounded by fibrous tissue.
Figures and Tables
Fig. 1
Lymphoepithelioma-like carcinoma of the pancreas head in a 76-year-old man.
A. Arterial phase image of abdominal CT shows an oval, well-defined, relatively homogeneous enhancement of the mass (white arrow) displacing adjacent vessel (black arrows).
B. In the delayed phase scan, a more heterogeneously enhanced mass (white arrow) is noted.
C. Axial T1-weighted gradient-recalled echo MR image shows a well-defined mass (white arrow) with intermediate to low signal intensity relative to the distal pancreas.
D. Axial T2-weighted turbo-spin echo MR image shows mass (white arrow) with high signal intensity relative to the distal pancreas.
E. Axial arterial-phase image of gadolinium-enhanced T1-weighted fat-suppressed gradient-recalled echo MR image shows relatively homogeneous enhancement and smooth contour of the mass (white arrow).
F. Axial five-minute delayed MR image reveals a more heterogeneous mass (white arrow) with bright rim- and septal enhanced portions (black arrows) and poorly enhanced nodular portions.
G. Cut surface of the gross specimen shows a well-circumscribed, lobulated and gray-white mass.
H. The nests (white arrows) consisted of tumor cells and lymphoid cells are surrounded by fibrous tissue (hematoxylin and eosin stain, × 40).
I. Photomicrograph of histologic specimen of the tumor shows atrophied pancreatic islets (black circles) in the periphery (hematoxylin and eosin stain, × 40).
J. Photomicrograph of tumor cells (black arrow) shows an arranged syncytial aggregates of lymphoplasma cells (gray arrow) (hematoxylin and eosin stain, × 200).
K. Tumor cells are positive for cytokeratin 19 (immunohistochemical stain, × 200).
References
1. Ishida M, Mori T, Shiomi H, Naka S, Tsujikawa T, Andoh A, et al. Non-Epstein-Barr virus associated lymphoepithelioma-like carcinoma of the inferior common bile duct. World J Gastrointest Oncol. 2011; 3:111–115.
2. Kekis PB, Murtin C, Künzli BM, Kappler A, Buchholz B, Büchler MW, et al. Epstein-Barr virus-associated lymphoepithelial carcinoma in the pancreas. Pancreas. 2004; 28:98–102.
3. Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990; 3:377–380.
4. Delaney D, Chetty R. Lymphoepithelioma-like carcinoma of the colon. Int J Clin Exp Pathol. 2012; 5:105–109.
5. Dinniwell R, Hanna WM, Mashhour M, Saad RS, Czarnota GJ. Lymphoepithelioma-like carcinoma of the breast: a diagnostic and therapeutic challenge. Curr Oncol. 2012; 19:e177–e183.
6. Williamson SR, Zhang S, Lopez-Beltran A, Shah RB, Montironi R, Tan PH, et al. Lymphoepithelioma-like carcinoma of the urinary bladder: clinicopathologic, immunohistochemical, and molecular features. Am J Surg Pathol. 2011; 35:474–483.
7. Val-Bernal JF, González-Márquez P, Ballestero R, Zubillaga S. Primary lymphoepithelioma-like carcinoma of the ureter. Ann Diagn Pathol. 2011; 15:218–220.
8. Kurihara K, Nagai H, Kasahara K, Kawai T, Saito K, Kanazawa K. Pleomorphic carcinoma of the pancreas with massive lymphocytic stromal infiltration and long-term survival after resection. Int J Pancreatol. 2000; 27:241–248.
9. Wilentz RE, Goggins M, Redston M, Marcus VA, Adsay NV, Sohn TA, et al. Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: a newly described and characterized entity. Am J Pathol. 2000; 156:1641–1651.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download