Abstract
Glufosinate ammonium (GLA) is a broad-spectrum herbicide used worldwide. Transient lesions involving the splenium of the corpus callosum have been described in patients with encephalitis or encephalopathy of varied etiology, but have not been reported in association with GLA poisoning. We describe a patient who had attempted suicide by ingesting GLA-containing herbicide, with a focal diffusion-restrictive lesion in the splenium of the corpus callosum on MRI, which had disappeared four weeks later with no sequalae.
Glufosinate ammonium (GLA) is a broad-spectrum herbicide used worldwide. As the use of GLA-containing herbicides has increased, poisoning from incidental and deliberate ingestion has also increased, and has been mostly reported from Japan. Most of glufosinate poisoning events were associated with suicide attempts using BASTA® (Bayer, Leverkusen, Germany). BASTA®, a herbicide containing glufosinate ammonium (18.5%) and the anionic surfactant, polyoxyethylene alkylether sulfate (30%), has been widely used in many countries since 1984 (1).
Glufosinate poisoning has been reported to induce central nervous system (CNS) symptoms, such as drowsiness, memory impairment, and seizures. Also, the included anionic surfactant, which increases blood vessel permeability, may cause symptoms such as reduced circulatory blood volume, altered cardiac function, and systemic peripheral vessel resistance (1). Periventricular white matter ischemias, hippocampal lesions, and both hippocampal and striatal lesions have been reported following GLA intoxication, together with radiological findings (2, 3). We describe a patient who developed a reversible callosal lesion by ingesting a GLA-containing herbicide in an attempted suicide.
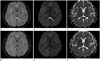
A 38-year-old woman was transferred to the emergency department with nausea, vomiting and sore throat, 3 hours after ingesting a GLA-containing herbicide in a suicide attempt. She had drunk 500 mL of BASTA®. Her initial vital signs were stable and she was mentally alert. Arterial blood gas analysis showed mild respiratory alkalosis. After gastric lavage followed by activated charcoal treatment, she underwent hemoperfusion. Four hours later, mild hypotension developed, with a blood pressure of 90/54, and she was admitted to the intensive care unit. A day later, she presented with drowsy mental status, a disoriented sense of time, and retrograde amnesia. Six days later, the drowsy mentality and disorientation had improved, but the retrograde and anterograde amnesia remained. Therefore, conventional and diffusion-weighted 3T MRI were performed. These revealed a well-demarcated, oval shaped lesion in the midline splenium of the corpus callosum, which showed high signal intensity on T2-weighted and diffusion images, low signal intensity on T1-weighted image, and no enhancement. The mean apparent diffusion coefficient (ADC) value of the splenial lesion of the corpus callosum was reduced to 324.5 ± 15.0 (10-5 mm2/s) (Fig. 1). No other brain parenchymal lesion was seen.
On the eleventh day of admission, the patient presented with improved mental status and general condition, and was discharged. Follow-up MRI, a month after the initial MRI examination, showed that the splenial lesion had disappeared.
Reversible lesions restricted to the splenium of the corpus callosum (Reversible Splenial Lesion Syndrome, or RESLES) have been described in patients with encephalitis or encephalopathy of varied etiology, including infection, high-altitude cerebral edema, antiepileptic drug therapy, and metabolic disturbances (4). However, RESLES associated with poisoning by a GLA-containing herbicide has not been reported.
The clinical presentation of reversible splenial lesion is non-specific. Instead, it is mainly dependent on the event that caused the condition, and most frequently manifests as an encephalopathy or encephalitis. Glufosinate intoxication has been reported to lead to memory impairment with or without hippocampal lesions (2, 3). Our patient displayed retrograde and anterograde amnesia that may have been associated with mild hippocampal injury that was not evident radiologically.
RESLES cases have been reported with a consistent pattern of neuroimaging abnormalities characterized by round lesions that are hyperintense on fluid attenuated inversion recovery and hypointense on T1-weighted sequences. Some of the splenial lesions showed restricted diffusion, with low ADC values in diffusion-weighted imaging, suggestive of cytotoxic edema (4).
The reversible splenial lesion in this case also presented as a diffusion-restricted lesion, probably indicative of cytotoxic edema, as in other reported cases of RESLES.
Glufosinatetory amino acid, and is thought to inhibit glutamine synthetase and glutamine decarboxylase, resulting in decreased glutamic acid levels. It may induce convulsions and memory impairment by interfering with the neurotransmitter function of endogenous glutamate (5). Glufosinate can induce neuronal excitatoxicity, which is known to cause neuronal cell death and produce degeneration of brain cells. Excitotoxic brain injury is considered a final common pathway for various neuropathologic conditions and causes cytotoxic edema (6) is a structural analogue of glutamic acid, a typical excita.
There is a report of a patient who showed mild encephalitis/encephalopathy with a reversible splenial lesion, and whose cerebrospinal fluid contained anti-glutamate ε2 receptor (GluRε2) antibodies (7).
Recently, autoantibodies against glutamate receptor ε2 have been detected in patients with epilepsia partialis continua, which is causally related to Rasmussen syndrome, nonparaneoplastic limbic encephalitis and acute encephalitis (8). And it is considered that glutamtate receptor autoantibodies are capable of inducing neuronal excitotoxicity (9). Glufosinate results in excitotoxicity and should have a similar effect to GluRε2 receptor antibodies. Hence glufosinate may play a similar role in the pathogenesis of reversible splenial injury as GluRε2 receptor antibodies.
Polyoxyethylene alkylether sulfate, the surface active agent in BASTA, increases the permeability of the blood-brain barrier, favoring access of glufosinate to the CNS and enhancing CNS toxicity, as shown in various animal and human studies (10).
We report glufosinate-containing herbicide intoxication can cause reversible splenial lesions. Glufosinate together with a surface active agent can induce cytotoxic edema in the splenium of the corpus callosum.
Figures and Tables
Fig. 1
MR images from a 38-year-old woman who ingested glufosinate ammonium-containing herbicide in a suicide attempt. Fluid attenuated inversion recovery (FLAIR) (A) and diffusion-weighted (B) axial MR images show an oval shaped high signal intensity lesion at the mid portion of splenium of the corpus callosum (arrows), which is low signal intensity (arrow) on the apparent diffusion coefficient (ADC) map (C), suggestive of cytotoxic edema. Follow-up axial MRI, one month later, shows complete resolution of the splenial lesion on FLAIR (D), diffusion-weighted MRI (E), and the ADC map (F).
References
1. Koyama K, Andou Y, Saruki K, Matsuo H. Delayed and severe toxicities of a herbicide containing glufosinate and a surfactant. Vet Hum Toxicol. 1994; 36:17–18.
2. Park HY, Lee PH, Shin DH, Kim GW. Anterograde amnesia with hippocampal lesions following glufosinate intoxication. Neurology. 2006; 67:914–915.
3. Lee HY, Song SY, Lee SH, Lee SY, Kim SH, Ryu SW. Vasogenic edema in striatum following ingestion of glufosinate-containing herbicide. J Clin Neurosci. 2009; 16:1372–1373.
4. Garcia-Monco JC, Cortina IE, Ferreira E, Martínez A, Ruiz L, Cabrera A, et al. Reversible splenial lesion syndrome (RESLES): what's in a name? J Neuroimaging. 2011; 21:e1–e14.
5. Nakaki T, Mishima A, Suzuki E, Shintani F, Fujii T. Glufosinate ammonium stimulates nitric oxide production through N-methyl D-aspartate receptors in rat cerebellum. Neurosci Lett. 2000; 290:209–212.
6. Moritani T, Smoker WR, Sato Y, Numaguchi Y, Westesson PL. Diffusion-weighted imaging of acute excitotoxic brain injury. AJNR Am J Neuroradiol. 2005; 26:216–228.
7. Fujiki Y, Nakajima H, Ito T, Kitaoka H, Takahashi Y. [A case of clinically mild encephalitis/encephalopathy with a reversible splenial lesion associated with anti-glutamate receptor antibody]. Rinsho Shinkeigaku. 2011; 51:510–513.
8. Takahashi Y, Kubota Y, Yamasaki E, Matsuda K. [Rasmussen encephalitis and non-herpetic acute limbic encephalitis]. Rinsho Shinkeigaku. 2008; 48:163–172.
9. Pleasure D. Diagnostic and pathogenic significance of glutamate receptor autoantibodies. Arch Neurol. 2008; 65:589–592.
10. Watanabe T, Sano T. Neurological effects of glufosinate poisoning with a brief review. Hum Exp Toxicol. 1998; 17:35–39.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download