Abstract
Pseudomyxoma peritonei (PMP) is an uncommon disease characterized by the seeding of mucin-secreting tumor cells throughout the abdomen and accumulation of mucin in the abdominal and pelvic cavities. Intraductal papillary mucinous neoplasms (IPMNs) of the pancreas are defined as pancreatic neoplasms that accumulate mucin within dilated ducts. Only a few cases of pancreatic IPMNs are associated with extra-pancreatic mucin and lead to PMP. This manuscript describes an unusual case of PMP caused by ruptured pancreatic IPMN.
Pseudomyxoma peritonei (PMP) is an uncommon disease characterized by the seeding of mucin-secreting tumor cells throughout the abdomen and accumulation of mucin in the abdominal and pelvic cavities (1). As first described in 1982 by Ohashi et al. (2), intraductal papillary mucinous neoplasms (IPMNs) are defined as pancreatic neoplasms that accumulate mucin within dilated ducts, and termedas "mucus-secreting pancreatic cancer". Seven cases of IPMNs have been associated with extra-pancreatic mucin and lead to the development of PMP, since the latter condition was first described in 1998 (3, 4, 5, 6, 7). This case report describes an unusual case of a patient with PMP caused by a ruptured IPMN.
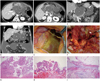
An 83-year-old female patient was admitted to our hospital with epigastric pain and vomiting for 3 days. Moreover, a physical examination performed in the emergency department revealed epigastric area tenderness. She had no remarkable history except hypertension and diabetes mellitus, and there was no history of chronic alcohol abuse. Multidetector computed tomography (MDCT) of the abdomen showed multiloculated cystic lesions in the lesser sac extending to the perigastric space (Fig. 1A-D). Additionally, a large amount of low-attenuation materials, which showed a slightly higher attenuation than that of urine, occupied the abdominal and pelvic cavities. The main pancreatic duct at the tail portion was markedly dilated and connected to the adjacent cystic lesion in the lesser sac. Additionally, a large defect that directly communicated with the low attenuated fluid collection in the perigastric space was found at the anterior portion of the cystic lesion in the lesser sac. No abnormality was found in the appendix. Thus, we diagnosed her with PMP, which was caused by a ruptured main duct type of IPMN. Additionally, based on the imaging features, we thought that the cystic lesions in the lesser sac represented a mucin pool by the ruptured dilated main duct.
Surgery was performed for the treatment of those lesions. During the surgery, a large amount of yellowish fluid and multiple gelatinous nodules covering the omentum (Fig. 1E), small bowel, colon and peritoneum were noted. Additionally, a large cystic lesion originating from the pancreas tail (Fig. 1F) was found, and the mucinous materials were spilled incidentally due to traumatic rupture during surgery. Distal pancreatectomy, omentectomy and debulking of mucin pool in the abdominal cavity were performed.
Grossly, the surgical specimen consisted of a ruptured, large, multilocular, thick-walled cystic lesion containing gelatinous material in the pancreatic tail. The attached omentum was thickened and mucinous nodules were attached to it.
Microscopically, the cystic lesion in lesser sac was lined by columnar mucinous epithelium covering the papillary fibrovascular cores, a finding that was compatible with this cystic lesion is dilating the main pancreatic duct itself (Fig. 1G). Most of the lining epithelium comprised tall columnar cells with nuclear stratification and small nucleoli. However, some cells showed architectural complexity with cribriforming and micropapillary formation, and the nuclei were round and lacked significant pseudostratification (Fig. 1H). No ovarian-like stroma or invasive component was noted. These histologic features were compatible with IPMN with high-grade dysplasia. Additionally, the omentum was partially dissected by lakes of mucin and scattered epithelial cells (Fig. 1I). These images were consistent with PMP.
PMP is a rare intra-abdominal neoplasm characterized by considerable mucin collection with implantation on the omentum and peritoneal surface. It originates most frequently from a ruptured adenoma or a well-differentiated mucinous adenocarcinoma of the appendix, ovary or colon (1). Moreover, PMP is rarely associated with mucinous carcinomas of the pancreas, such as mucinous cystic neoplasms and IPMNs (5).
IPMN is a type of tumor that grows within the pancreatic ducts and is characterized by the production of mucinous materials (2). IPMNs are neoplasms with tall, columnar, mucin-containing epithelium with or without papillary proliferations and extensively involving the main or side pancreatic branches without ovarian-like stroma, representing mucinous cystic neoplasms (8).
Although the overproduction of mucus is characteristic of IPMN, extra-pancreatic mucin--such as that associated with PMP--has been reported in only seven cases since IPMNs were first described in 1998, including a case of leaked mucinous material from a ruptured cystic mass by acute pancreatitis (3, 4, 5, 6, 7).
Our MDCT images showed dilatation of the main pancreatic duct at the tail portion without evidence of an obstructive lesion at the proximal portion. Thus, we could diagnose a main duct type of IPMN. Additionally, a markedly dilated proximal main pancreatic duct was directly communicating with neighboring cystic lesions in the lesser sac. Thus, we believed that the dilated main duct was ruptured at the proximal portion, resulting in formation of multiloculated mucinous cystic lesions in the lesser sac. However, pathologically, cystic lesions in the lesser sac were compatible with the tremendously dilated main pancreatic duct itself, which showed features of IPMN and was observed to be perforated at the upper margin of the cystic lesion than connected with low attenuated fluid collection in perigastric area.
MDCT is now widely used for the diagnosis of PMP. Many characteristic findings such as scalloping of the liver, spleen and mesentery can be seen (9). However, marked similarities remain in the CT findings of normal ascites and PMP. Curvilinear calcifications, omental thickening and multiple septations in the ascitic fluid might be suggestive of PMP (9). However, our case showed only a massive amount of fluid collection in the abdominal and pelvic cavities without the above-mentioned typical findings of PMP. Thus, definite differentiation between simple ascites and PMP could be challenging. However, we made the diagnosis of PMP because the images of our case showed typical findings of the ruptured main duct type of IPMN.
In 2006, consensus guidelines for the treatment of IPMN were established, and the current recommendations are to resect all the main duct, combined and symptomatic branch duct types of IPMN (10). Although no guidelines for the treatment of IPMN with the presence of PMP, initial treatment for PMP included radical, debulking and cytoreductive surgery. Presently, many alternative treatments can be used to treat IPMN, such as intraperitoneal chemotherapy and radiotherapy (7). Our patient had undergone distal pancreatectomy for IPMN because there was no evidence of an abnormal finding at the resection margin on frozen section biopsy during surgery; additionally, debulking surgery was performed for PMP in the abdominal cavity.
IPMN shows a better 5-year survival rate than that typically reported for invasive ductal adenocarcinoma of the pancreas (8). Additionally, the clinical course of PMP may demonstrate an overall poor prognosis because of recurrence, even with aggressive treatments (7). Previously reported cases (4, 5, 7) showed a survival duration of 6 to 48 months after surgery. However, our case showed a considerably greater amount of mucinous material occupying almost the entire abdominal cavity and multiple mucinous nodules attached to the omentum, small bowel, colon and peritoneum. Thus, we expect the prognosis of our case to be worse than that of cases reported previously.
In conclusion, we report a case of PMP caused by a ruptured IPMN of the pancreas, and radiologists need to consider IPMN of the pancreas to be a possible cause of PMP.
Figures and Tables
Fig. 1
An 83-year-old female with pseudomyxoma peritonei caused by a ruptured intraductal papillary mucinous neoplasm. Multidetector computed tomography of abdomen axial (A, B), curved multiplanar reconstruction (C) and coronal reformatted (D) images showed multiloculated cystic lesions in the lesser sac (*) extending to the low attenuated fluid collection in perigastric (**) region. Additionally, a large amount of low-density materials, which exhibited a slightly higher density than that of urine, was present in the abdomen and pelvic cavities. The main pancreatic duct (arrowhead) in the pancreatic tail was markedly dilated and continued to the cystic lesion in the lesser sac (*). Additionally, a large defect in the cystic wall that directly communicated to the fluid collection in the perigastric space was observed at the anterior portion (thin arrow). During the surgery, a large amount of yellowish fluid and multiple gelatinous nodules attached to the omentum were observed (E). Additionally, a large cystic mass originating from the pancreas tail (F) was noted, and the mucinous materials spilled incidentally (thick arrow) due to traumatic rupture during surgery. On pathologic examination, the pancreatic cystic mass was filled with various complex papillary projections lined by tall columnar epithelial cells [hematoxylin and eosin (H&E) staining; magnification, × 20] (G). The lining epithelium showed architectural complexity with cribriforming and micropapillary formation, and the nuclei were round and lacked significant pseudostratification (H&E staining; magnification, × 200) (H). Additionally, the omentum revealed abundant pools of mucoid material with collections of inflammatory cells and hemorrhagic foci (H&E staining; magnification, × 40) (I).
References
1. van Ruth S, Acherman YI, van de Vijver MJ, Hart AA, Verwaal VJ, Zoetmulder FA. Pseudomyxoma peritonei: a review of 62 cases. Eur J Surg Oncol. 2003; 29:682–688.
2. Ohashi K, Murakami Y, Maruyama M, Takekoshi T, Ohta H, Ohashi I. Four cases of mucous secreting pancreatic cancer. Prog Dig Endosc. 1982; 20:348–351.
3. Zanelli M, Casadei R, Santini D, Gallo C, Verdirame F, La Donna M, et al. Pseudomyxoma peritonei associated with intraductal papillary-mucinous neoplasm of the pancreas. Pancreas. 1998; 17:100–102.
4. Imaoka H, Yamao K, Salem AA, Mizuno N, Takahashi K, Sawaki A, et al. Pseudomyxoma peritonei caused by acute pancreatitis in intraductal papillary mucinous carcinoma of the pancreas. Pancreas. 2006; 32:223–224.
5. Mizuta Y, Akazawa Y, Shiozawa K, Ohara H, Ohba K, Ohnita K, et al. Pseudomyxoma peritonei accompanied by intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2005; 5:470–474.
6. Nepka Ch, Potamianos S, Karadana M, Barbanis S, Kapsoritakis A, Koukoulis G. Ascitic fluid cytology in a rare case of pseudomyxoma peritonei originating from intraductal papillary mucinous neoplasm of the pancreas. Cytopathology. 2009; 20:271–273.
7. Rosenberger LH, Stein LH, Witkiewicz AK, Kennedy EP, Yeo CJ. Intraductal papillary mucinous neoplasm (IPMN) with extra-pancreatic mucin: a case series and review of the literature. J Gastrointest Surg. 2012; 16:762–770.
8. Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004; 351:1218–1226.
9. Hinson FL, Ambrose NS. Pseudomyxoma peritonei. Br J Surg. 1998; 85:1332–1339.
10. Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006; 6:17–32.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download