Abstract
Purpose
This study retrospectively compared the accuracy of angiographic sentinel signs (sentinel vessels, hypovascular areas, and delayed dots) with extravasation in the diagnosis of ruptured hepatocellular carcinoma (HCC).
Materials and Methods
Sixteen patients diagnosed with HCC between March 2007 and November 2011 were evaluated. Among the patients, we identified 32 HCCs (19 ruptured, 13 unruptured), and assessed all HCCs by hepatic angiography with regard to extravasation, sentinel vessels, hypovascular areas, and delayed dots. We compared the sensitivity and specificity of the sentinel signs with those of the extravasation for the diagnosis of a ruptured HCC.
Results
For the angiographic diagnosis of a ruptured HCC, the sensitivity of the sentinel signs (sentinel vessel, 63.2%; hypovascular area, 89.5%; delayed dot, 72.7%) was higher than the sensitivity of extravasation (15.8%). The difference in sensitivity between each sentinel sign and extravasation was statistically significant (sentinel vessel, p = 0.012; hypovascular area, p < 0.001; delayed dot, p = 0.039). The specificity of sentinel signs for the diagnosis of ruptured HCC was not statistically different from the specificity of extravasation.
Hepatocellular carcinoma (HCC) rupture is the most common non-traumatic cause of hepatic hemorrhage (1, 2), and it occurs in approximately 3% to 26% of patients with HCC (3, 4, 5, 6, 7, 8). Due to its high in-hospital mortality rate (51-67%), the prompt diagnosis and treatment of spontaneous HCC rupture is critical (8, 9). Transcatheter arterial embolization (TAE) is the preferred method of establishing hemostasis (10, 11), and the primary goal of the overall management for a patient with a ruptured HCC is to obtain hemostasis while preserving as much liver function as possible (3, 10, 11, 12). In order to preserve liver function, tumor-specific, selective hepatic artery embolization is mandatory. However, selective embolization is difficult, because the only hepatic angiographic sign of a ruptured HCC, extravasation, lacks diagnostic sensitivity (13.2-35.7%) (13). In contrast, a ruptured HCC demonstrates many findings on computed tomography (CT), such as active extravasation of contrast, discontinuity of the hepatic surface, subcapsular hematoma, enucleation, and hemoperitoneum (3, 4, 13, 14). Therefore, in order to identify a ruptured HCC, hepatic angiographic findings must be compared with CT findings. However, this is more difficult when multiple HCC tumor foci are present in the same patient.
Several studies have suggested that HCC rupture is caused by the disruption of friable feeding arteries, leading to hemorrhage and increased intra-tumoral pressure from the local bleed (8, 9, 14, 15, 16, 17, 18). During hepatic angiography, we have previously observed that ruptured HCCs are associated with disproportionately dilated and distorted blood vessels, surrounding hypovascular areas and dot-like residual contrast stains during the delayed phase of the angiogram. Therefore, we hypothesized that disproportionately dilated and distorted blood vessels, hypovascular areas and dot-like residual contrast stains can be used to enhance the detection of a ruptured HCC during hepatic angiography.
In order to test this hypothesis, the present study retrospectively compared the sensitivity and specificity of these novel "sentinel" signs with extravasation, for the angiographic diagnosis of a ruptured HCC. This study also assessed for the differences in size and location between ruptured and unruptured HCCs.
The current study was approved by our institutional review board, and the requirement for patient consent for this retrospective analysis was waived. Fig. 1 shows flow chart of patient selection. At our institution, HCC was diagnosed in 274 patients (894 cases) between March 2007 and November 2011, on the basis of CT, magnetic resonance imaging, ultrasound, and alpha-fetoprotein or biopsy. Among 274 patients, the inclusion criteria were as follows: 1) diagnosed HCC rupture on CT; 2) performed initial TAE for treatment; and 3) obtained sufficient hepatic angiographic images. The patients satisfying all three conditions were included. The patients not satisfying any one of the conditions were excluded. We selected twenty four patients (26 cases) with HCC rupture on CT, in consideration of previous reports (3, 4, 13, 14). Seven patients (7 cases) were excluded, in whom TAE were not performed. One patient (1 case) was excluded due to insufficient image. Two cases were also excluded because these were follow-up hepatic angiography.
Finally, sixteen patients (16 cases) were enrolled in our study [14 men, 2 women; mean age (± standard deviation) 55.9 ± 12.7 years; range 37-74 years].
The CT and hepatic angiographic images of the sixteen patients were retrospectively reviewed, and the findings were agreed upon by two radiologists (D.H.N. and S.J.Y.). We included HCCs greater than 2 cm in maximal diameter on CT, as tumors less than 2 cm are difficult to correlate with hepatic angiographic findings. Among the sixteen patients, a total of 32 HCCs were identified: 19 HCCs were ruptured; and 13 HCCs were unruptured. HCC size and location were assessed by CT: tumor size was defined as the maximal tumor diameter on axial images; and tumor location was assigned according to the Couinaud classification.
Several CT findings were used to identify a ruptured HCC, including hemoperitoneum, extravasation, discontinuity of the hepatic surface, and enucleation. On hepatic angiography, extravasation, sentinel vessels, hypovascular areas, and delayed dots were assessed. The definition of extravasation is a leakage of contrast material from HCCs and contrast spillage off the peritoneum during hepatic angiography. Sentinel vessels are disproportionately dilated and distorted blood vessels, compared to adjacent hepatic arteries (Fig. 2). Hypovascular areas are areas of HCC that lack tumor stains. Delayed dots are dot-like residual contrast stains present after washout of most tumor stains during hepatic angiography (Fig. 3). We coined the term "sentinel signs" for summation of these three new findings (sentinel vessels, hypovascular areas, and delayed dots).
First, after puncturing the right femoral artery with the Seldinger technique, superior mesenteric arteriography was performed using 5 Fr RH (Cook, Bloomington, IN, USA) catheter to visualize the portal circulation. Then a 5 Fr RH catheter was placed in the proper hepatic artery; and hepatic angiography was performed to evaluate surrounding vascular anatomy and artery of bleeding focus or blood supply. Next, a 2.0 Fr or 2.2 Fr (Cook, Bloomington, IN, USA) microcatheter was inserted into the catheter (which remained in the proper hepatic artery), and was advanced into the artery of bleeding focus or blood supply. Following this, embolization of the artery of bleeding focus or blood supply was performed with gelatin sponge particles (Gel-part; Nippon Kayaku, Tokyo, Japan), which had been crushed into approximately 0.2-0.5 mm particles by pumping with a three-way stopcock valve and two 1 mL syringes. Finally, follow-up angiography was performed immediately after TAE.
All statistical analyses were performed with statistical software (PASW, version 18.0, SPSS Inc., Chicago, IL, USA). Continuous variables are presented as mean ± standard deviation, and they were compared using the student's t-test for independent variables. Categorical variables are presented as frequencies and percentages, and they were compared using a chi-square test or a Fisher's exact test. For the comparisons of sensitivity and specificity between imaging findings, the Cochran's Q test was used. Cochran's Q test is an extension of the McNemar test for related samples that assesses differences between three or more matched sets of frequencies or proportions. When the Cochran's Q test was positive (p < 0.05), post hoc analysis was used to examine the differences between each categorical variable.
Of the 19 ruptured HCCs, 14 were located in the right hepatic lobe (segment 5, n = 1; segment 6, n = 2; segment 7, n = 1; segment 8, n = 2; segment 5 and 6, n = 2; segment 6 and 7, n = 2; segment 5 and 8, n = 1; segment 7 and 8, n = 1; segment 5, 6, and 8, n = 2). Two were located in the left hepatic lobe (segment 4, n = 2), and 3 were located in the caudate lobe. Of the remaining 13 unruptured HCCs, 8 were located in the right hepatic lobe (segment 5, n = 1; segment 6, n = 1; segment 7, n = 4; segment 8, n = 1; segment 6 and 7, n = 1), and 5 were located in the left hepatic lobe (segment 2, n = 2; segment 3, n = 2; segment 4, n = 1). The locations of ruptured and unruptured HCCs were not statistically different (p = 0.734). The mean size of ruptured HCCs was 9.0 ± 3.5 cm (range, 2.9-16.8 cm), while the mean size of unruptured HCCs was 3.4 ± 1.8 cm (range, 2.0-7.4 cm). Ruptured HCCs were significantly larger than unruptured HCCs (p < 0.05) (Table 1).
The mean time-interval between pretreatment abdominal CT and hepatic angiography was 13.9 ± 23.7 hours (range, 1-72 hours). We obtained a delayed phase hepatic angiogram after full tumor washout in 23 of the 32 HCCs; and the mean hepatic angiogram acquisition time for these 23 HCCs was 16.2 ± 5.0 seconds (range, 10-21 seconds). In the nine patients for whom delayed phase hepatic angiograms were not obtained, the mean hepatic angiogram acquisition time was 7.9 ± 1.5 seconds (range, 8-11 seconds). Overall, 11 ruptured and 12 unruptured HCCs were evaluated for delayed dots on hepatic angiography.
The CT findings of ruptured HCCs included extravasation in 7/19 patients (36.8%), discontinuity of the hepatic surface in 16/19 patients (84.2%), enucleation in 7/19 patients (36.8%), and hemoperitoneum in 19/19 patients (100%). The hepatic angiographic findings of ruptured HCCs included extravasation of contrast material in 3/19 patients (15.8%), a sentinel vessel in 12/19 patients (63.2%), a hypovascular area in 17/19 patients (89.5%), and a delayed dot in 8/11 patients (72.7%). Hepatic angiography in unruptured HCCs showed a sentinel vessel in 2/13 patients (15.4%), a hypovascular area in 2/13 patients (15.4%), and a delayed dot in 1/9 patients (9.1%). The frequencies of finding a sentinel vessel, hypovascular area, and delayed dot were significantly different between ruptured and unruptured HCCs (sentinel vessel, p = 0.021; hypovascular area, p < 0.001; delayed dot, p = 0.002).
The sensitivity of extravasation, sentinel vessels, hypovascular areas, and delayed dots in the diagnosis of a ruptured HCC was 15.8%, 63.2%, 89.5%, and 72.7%, respectively. The specificity of extravasation, sentinel vessels, hypovascular areas, and delayed dots in the diagnosis of a ruptured HCC was 100%, 84.6%, 84.6%, and 91.7%, respectively.
The sensitivities of the sentinel signs for the diagnosis of a ruptured HCC were higher than the sensitivity of extravasation (15.8%); and the sensitivity of each sentinel sign was significantly different compared to the sensitivity of extravasation (sentinel vessel, p = 0.012; hypovascular area, p < 0.001; delayed dot, p = 0.039, Cochran's Q test with post hoc analysis). The specificities of the sentinel signs for the diagnosis of a ruptured HCC were not significantly different from the specificity of extravasation (p = 1.000, Cochran's Q test) (Table 2).
In this study, we compared the accuracy of the hepatic angiographic findings of extravasation with "sentinel signs" (sentinel vessels, hypovascular areas, and delayed dots) for the diagnosis of a ruptured HCC. We found that sentinel signs have a higher sensitivity and comparable specificity relative to extravasation for the diagnosis of a ruptured HCC.
The results of our study may be due to the mechanism of HCC rupture, although the mechanism of spontaneous HCC rupture is not fully known. A more convincing hypothesis is a sudden increase in intra-tumoral pressure, followed by bursting of the tumor surface (19). Increased intra-tumoral pressure may be caused by vascular invasion with venous obstruction (14) or vascular injury (19, 20). The portal vein and subcapsular collaterals are the main venous drainage channels of HCCs. In the presence of portal hypertension or portal vein thrombosis and hepatic vein occlusion by the HCC, venous drainage is completely blocked and the resultant venous congestion leads to a rapid increase in intra-tumoral pressure and HCC rupture (19, 21). Recently, Zhu et al. (22, 23, 24, 25, 26) reported that vascular injury may be the main cause of HCC rupture. In this scenario, poorly functioning macrophage phagocytosis results in accumulation of immune complexes composed of hepatitis B virus e1 antigen (HBeAg/1), complement C1q, and immunoglobulins in the elastic membrane of arteries within the HCC (22), and subsequent neutrophilic infiltration into the vascular wall causes vascular injury (26). This vascular injury includes elastin proliferation, collagenase expression and collagen fibril degradation, which causes stiffening and weakening of already injured vessel walls. In this setting, the capability of the vessel wall to resist stretching decreases, and the vessels readily split, causing hemorrhage and hematoma within the tumor, followed by tumor rupture (20, 23, 26). Based on this hypothesis, the sentinel signs described herein would be developed by the splitting and expansion of injured feeding arteries (sentinel vessels), which lead to extravasation of arterial blood into perivascular areas within the HCC (hypovascular areas and delayed dots). The increased intra-tumoral pressure may lead to hepatic vein occlusion, venous congestion, and tumor infarction. The venous infarction due to high intra-tumoral pressure compresses the surrounding liver parenchyma and leads to disruption of the liver parenchyma at its weakest point at the interface of the hepatic surface and peritoneum. According to this scenario, extravasation may be the final step of HCC rupture; however, the sentinel signs can be noted even before the rupture (Table 3).
The rate of HCC re-rupture after TAE is 25%, and patients that suffer re-rupture have a poor prognosis (27). Therefore, the diagnosis of impending HCC rupture is just as important as the detection of already ruptured HCC. However, there are no studies that attempt to use hepatic angiographic findings to identify impending HCC rupture. In this study, an HCC that had not been ruptured at the time of the initial embolization due to a ruptured HCC was found to be ruptured one month later. In a retrospective review of the tumor, there was a sentinel vessel in the tumor on hepatic angiography. Unfortunately, the hepatic angiogram showed only half of the tumor due to field of view limitations (a ruptured tumor was located in the right lobe, and an unruptured tumor was located in the left lobe). Therefore, we could not fully evaluate the mass for other sentinel signs (hypovascular areas or delayed dots). Nevertheless, this example suggests that a sentinel vessel may be a sign of impending HCC rupture. Zhu et al. (25) found evidence of vascular injury in not only ruptured HCCs, but in two specimens of unruptured HCC.
A delayed dot refers to a contrast stain that remains after hepatic artery and tumor washout. A small amount of contrast material in the liver on CT after a traumatic injury may represent pseudoaneurysm formation or extravasated contrast due to arterial injury (2). Angiographic tumor staining or pooling of contrast media is closely related to dilated and crowded blood spaces (21). Based on these findings, a delayed dot may represent extravasation of contrast or pseudoaneurysm formation related to injured feeding arteries within an HCC. However, in this study, we were only able to obtain delayed hepatic angiograms of more than 11 or 12 seconds in some patients, which limited our evaluation of the sensitivity of the finding of a delayed dot. If we obtained delayed hepatic angiograms of more than 12 seconds in all patients, the sensitivity of the delayed dot may have been higher than what we found. Therefore, delayed imaging acquisition of more than 12 seconds is required in the future.
Most of the ruptured HCCs demonstrated one or two sentinel signs, but sentinel signs were not applicable to HCCs located in the caudate lobe of the liver. The three ruptured HCCs located in the caudate lobe did not show a sentinel vessel. Moreover, one of the ruptured HCCs located in the caudate lobe showed neither a sentinel sign nor extravasation. For this patient, the only diagnostic sign of a ruptured HCC was hemoperitoneum on CT. The difficulty in diagnosing this patient's ruptured HCC may have been due to the small amount of injected contrast material and the small size of the mass. HCCs located in the caudate lobe are supplied by the caudate artery, which can be derived from the right or left hepatic artery, both hepatic arteries and the common or proper hepatic artery (28). Therefore, in the absence of highly selective hepatic arteriograms, the amount of contrast material that reaches HCCs in the caudate lobe is likely to be insufficient to demonstrate features of HCC rupture. The small size of the caudate lobe mass was likely another factor that made identification of rupture difficult, because small tumor size coupled with insufficient contrast injection can result in suboptimal tumor visualization. The mean size of the three ruptured HCCs located in the caudate lobe was 5.6 cm, which was smaller than the mean size of the ruptured HCCs (9.0 cm) that were located in the right and left lobes. Normal hepatic parenchyma surrounding HCC can protect tumors from rupture. Conversely, the thinner the normal hepatic parenchymal wall, the more easily the HCC can rupture. If an HCC is located in the central part of the liver, the mass must grow large enough to reach the hepatic surface in order to rupture. However, if an HCC is located at the liver periphery or in the caudate lobe, it is able to rupture earlier and at a smaller size compared to centrally located HCC. When an HCC is small, angiographic changes suggesting vessel injury or intra-tumoral hemorrhage may be minimal or undetectable. For identifying and detecting vessels, cone beam CT was useful during hepatic angiography (29). Therefore, we speculate cone beam CT is an alternative method to detect a small ruptured HCC.
Our study has several limitations. First, a relatively small patient population was used to study the diagnostic performance of sentinel signs compared to extravasation. Second, CT and hepatic angiography were obtained at varying time intervals. The time interval can affect the sensitivity of extravasation, with shorter time intervals contributing to the low sensitivity of the finding of contrast leakage. Third, we could not correlate the sentinel signs with pathologic findings of a ruptured HCC, and we could not confirm whether the sentinel vessels were occluded hepatic veins or injured feeding arteries. We presumed that the sentinel vessels were injured feeding arteries, based on previous evidence of vascular injury in ruptured HCCs (17, 21, 22, 23, 24). However, Chearanai et al. (21) has provided an example of a patient with a dilated, irregularly saccular, and obstructed vessel that turned out to be a hepatic vein in the setting of a ruptured HCC.
In conclusion, hepatic angiographic sentinel signs (sentinel vessels, hypovascular areas, and delayed dots) are superior to extravasation for the detection of a ruptured HCC. In addition, delayed hepatic angiographic time of more than 12 seconds is required to increase the detection of ruptured HCCs. Although some HCCs with sentinel signs were not ruptured, the sentinel signs may indicate impending rupture, and hence embolization of HCCs with sentinel signs without extravasation may provide a survival benefit. Future studies in larger patient populations are needed to confirm whether these novel findings can be used to predict HCC rupture.
Figures and Tables
 | Fig. 1Flow chart of patient selection.
Note.-HCC = hepatocellular carcinoma, TAE = transcatheter arterial embolization
|
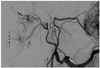 | Fig. 2Sentinel vessel. A 50-year-old male with hepatocellular carcinoma rupture at liver segment 5. On hepatic angiogram, abnormal dilatated and tortuous peripheral vessels (arrows) are seen in comparison with proximal segmental vessels. |
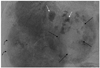 | Fig. 3Surrounding hypovascular area and delayed dot. A 64-year-old male with hepatocellular carcinoma (HCC) ruptured liver segment 4. Hepatic angiogram shows a barely staining area (black straight arrows) in hypervascular stain and dot like residual contrast stains (white straight arrows). This is different from usual HCCs which show hypervascular staining (curved arrows). |
References
1. Casillas VJ, Amendola MA, Gascue A, Pinnar N, Levi JU, Perez JM. Imaging of nontraumatic hemorrhagic hepatic lesions. Radiographics. 2000; 20:367–378.
2. Lubner M, Menias C, Rucker C, Bhalla S, Peterson CM, Wang L, et al. Blood in the belly: CT findings of hemoperitoneum. Radiographics. 2007; 27:109–125.
3. Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006; 141:191–198.
4. Choi BG, Park SH, Byun JY, Jung SE, Choi KH, Han JY. The findings of ruptured hepatocellular carcinoma on helical CT. Br J Radiol. 2001; 74:142–146.
5. Miyoshi A, Kitahara K, Kohya N, Noshiro H, Miyazahi K. Outcomes of patients with spontaneous rupture of hepatocellular carcinoma. Hepatogastroenterology. 2011; 58:99–102.
6. Al-Mashat FM, Sibiany AM, Kashgari RH, Maimani AA, Al-Radi AO, Balawy IA, et al. Spontaneous rupture of hepatocellular carcinoma. Saudi Med J. 2002; 23:866–870.
7. Liu CL, Fan ST, Lo CM, Tso WK, Poon RT, Lam CM, et al. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001; 19:3725–3732.
8. Chen CY, Lin XZ, Shin JS, Lin CY, Leow TC, Chen CY, et al. Spontaneous rupture of hepatocellular carcinoma. A review of 141 Taiwanese cases and comparison with non-rupture cases. J Clin Gastroenterol. 1995; 21:238–242.
9. Leung KL, Lau WY, Lai PB, Yiu RY, Meng WC, Leow CK. Spontaneous rupture of hepatocellular carcinoma: conservative management and selective intervention. Arch Surg. 1999; 134:1103–1107.
10. Ngan H, Tso WK, Lai CL, Fan ST. The role of hepatic arterial embolization in the treatment of spontaneous rupture of hepatocellular carcinoma. Clin Radiol. 1998; 53:338–341.
11. Corr P, Chan M, Lau WY, Metreweli C. The role of hepatic arterial embolization in the management of ruptured hepatocellular carcinoma. Clin Radiol. 1993; 48:163–165.
12. Battula N, Madanur M, Priest O, Srinivasan P, O'Grady J, Heneghan MA, et al. Spontaneous rupture of hepatocellular carcinoma: a Western experience. Am J Surg. 2009; 197:164–167.
13. Kim HC, Yang DM, Jin W, Park SJ. The various manifestations of ruptured hepatocellular carcinoma: CT imaging findings. Abdom Imaging. 2008; 33:633–642.
14. Kim PT, Su JC, Buczkowski AK, Schaeffer DF, Chung SW, Scudamore CH, et al. Computed tomography and angiographic interventional features of ruptured hepatocellular carcinoma: pictorial essay. Can Assoc Radiol J. 2006; 57:159–168.
15. Tarantino L, Sordelli I, Calise F, Ripa C, Perrotta M, Sperlongano P. Prognosis of patients with spontaneous rupture of hepatocellular carcinoma in cirrhosis. Updates Surg. 2011; 63:25–30.
16. Ong GB, Taw JL. Spontaneous rupture of hepatocellular carcinoma. Br Med J. 1972; 4:146–149.
17. Kim PN, Kim IY, Bae WK, Lee BH. Computed tomographic findings of ruptured hepatic malignancy. Gastrointest Radiol. 1991; 16:334–336.
18. Hermann RE, David TE. Spontaneous rupture of the liver caused by hepatomas. Surgery. 1973; 74:715–719.
19. Zhu LX, Wang GS, Fan ST. Spontaneous rupture of hepatocellular carcinoma. Br J Surg. 1996; 83:602–607.
20. Veltchev LM. Spontaneous rupture of hepatocellular carcinoma and hemoperitoneum-management and long term survival. J IMAB. 2009; 15:53–57.
21. Chearanai O, Plengvanit U, Asavanich C, Damrongsak D, Sindhvananda K, Boonyapisit S. Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer. 1983; 51:1532–1536.
22. Zhu LX, Geng XP, Fan SD. [Mechanism of spontaneous rupture of hepatocellular carcinoma]. Zhonghua Wai Ke Za Zhi. 2004; 42:1036–1039.
23. Zhu LX, Meng XL, Fan ST. Elasticity of small artery in patient with spontaneous rupture of hepatocellular carcinoma. Hepatol Res. 2004; 29:13–17.
24. Zhu LX, Liu Y, Fan ST. Ultrastructural study of the vascular endothelium of patients with spontaneous rupture of hepatocellular carcinoma. Asian J Surg. 2002; 25:157–162.
25. Zhu LX, Geng XP, Fan ST. Spontaneous rupture of hepatocellular carcinoma and vascular injury. Arch Surg. 2001; 136:682–687.
26. Zhu LX, Geng XP, Fan ST. Relationship between vascular elasticity and spontaneous rupture of hepatocellular carcinoma. The Chin-Ger J Clin Oncol. 2003; 2:18–22.
27. Soyer P, Van Beers B, Goffette P, Zeitoun G, Pringot J, Leves-que M. [The role of embolization and chemo-embolization in the emergency treatment of hemoperitoneum caused by spontaneous rupture of hepatocellular carcinoma]. Gastroenterol Clin Biol. 1993; 17:643–648.
28. Shibata T, Maetani Y, Ametani F, Kubo T, Itoh K, Konishi J. Efficacy of nonsurgical treatments for hepatocellular carcinoma in the caudate lobe. Cardiovasc Intervent Radiol. 2002; 25:186–192.
29. Miyayama S, Yamashiro M, Hashimoto M, Hashimoto N, Ikuno M, Okumura K, et al. Identification of small hepatocellular carcinoma and tumor-feeding branches with cone-beam CT guidance technology during transcatheter arterial chemoembolization. J Vasc Interv Radiol. 2013; 24:501–508.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download