Abstract
We report a case of a mycotic pulmonary aneurysm associated with Candida endocarditis in a 53-year-old male with lymphoma. The initial diagnosis was a pulmonary artery aneurysm attributable to vasculitis, such as that associated with Behçet's disease, but a mycotic pulmonary artery aneurysm was later considered as a differential diagnosis. Identification of valve vegetation on the chest CT was helpful in this regard. We review the literature on the disease etiology, radiological findings, and management options.
Infectious or mycotic pulmonary artery aneurysms (PAAs) are rare vascular abnormalities triggered by a variety of microorganisms, particularly of the bacteria including Staphylococcus aureus and Streptococcus species, and rarely by fungi such as Aspergillus and Candida species (1). PAAs are often associated with infective endocarditis or pneumonia, and PAA patients suffer high mortality (2). Early diagnosis and prompt intervention are important to prevent life-threatening hemorrhage (3). However, the diagnosis of a mycotic pulmonary artery aneurysm can be difficult in the absence of pneumonic consolidation adjacent to the aneurysm, or cavitary nodules which are suggestive of septic embolism.
We present a case of an infectious pulmonary arterial aneurysm with Candida endocarditis in a patient initially diagnosed with vasculitis. We review the literature on the etiology, radiologic findings, and management of the condition.
A 53-year-old male presented at our hospital complaining of chills, cold sweats, and dyspnea with 15 days in duration. He had a history of Hodgkin's lymphoma, and had been treated with 12 courses of ABVD chemotherapy (doxorubicin, bleomycin, vinblastine, and acarbazine) at a local hospital. One year ago, he achieved complete remission. On admission, he complained of tachycardia (102 bpm). Laboratory workup revealed an elevated C-reactive protein level (23.59 mg/dL; normal < 0.5 mg/dL) and neutrophilia (11200 neutrophils/µL; normal 4000-11000/µL). However, blood cultures were negative for both bacteria and fungi.
Chest computed tomography (CT) was performed before and after administration of intravenous contrast material. The axial CT image showed multiple aneurysmal dilatation of the pulmonary arteries, with or without thrombi. No adjacent pneumonic consolidation or cavitary nodulation was evident in either lung (Fig. 1A-C). CT venography of the lower extremities yielded no evidence of deep vein thrombosis. The aneurysms were thought to be manifestations of vasculitis such as that associated with Behçet's disease. However, our patient had no oral ulcer nor history of recurrent genital ulceration, uveitis, skin lesion, and yielded a negative skin pathogen test result. Further, antineutrophil cytoplasmic antibodies and antinuclear antibody data were also negative. His symptoms improved after the treatments with antibiotics, heparin, and warfarin, and he was discharged.
Eight days after discharge, he returned to our hospital complaining of aggravated symptoms and was admitted for further evaluation. A repeated chest CT scan revealed a lobulated poorly attenuated mass of 2.4 cm in diameter on the tricuspid valve, suggestive of the presence of vegetation (Fig. 1C). The aneurysms in the right and left inferior pulmonary arteries and the thrombi in the left inferior pulmonary artery remained unchanged. A necrotic consolidation in the right lower lobe, suggestive of pulmonary infarction, was also noted (Fig. 1D). Echocardiography was performed the next day for further evaluation of the cardiac mass. A highly mobile echogenic round mass was evident on the destroyed posterior/septal leaflet of the tricuspid valve. The mass was 2.3 × 2.3 cm in size, and another 1.6 × 0.8 cm sized oval echogenic mass was also noted on the anterior leaflet of the tricuspid valve. The masses were considered to be either vegetative in nature, or to be cardiac lymphoma metastases. However, Candida albicans was cultured from blood. We made a diagnosis of infective endocarditis caused by Candida albicans, associated with mycotic pulmonary artery aneurysms and septic pulmonary emboli. Intravenous amphotericin was commenced, but surgery was required to treat persistent pulmonary embolism and the continued presence of mobile vegetation of over 10 mm in diameter (4).
The operation was performed in a standard manner, featuring cardiac arrest and use of a cardiopulmonary bypass. When the right atrium was opened, large vegetation was found to be attached to the destroyed tricuspid valve (Fig. 1E). The vegetation and the destroyed valve were excised, and a 31-mm Carpentier-Edwards pericardial bioprosthesis (Edwards Lifesciences, Irvine, CA, USA) was fitted. Pathologic examination confirmed the presence of infective endocarditis caused by a Candida species (Fig. 1F). Aneurysmectomy was not used to repair the mycotic pulmonary aneurysm.
During the following week, the patient's clinical condition improved greatly and blood cultures became negative. However, a follow-up chest CT scan taken 3 months after the operation revealed no significant change in the mycotic PAA or septic pulmonary thrombi, despite the prescription of fluconazole. The patient died 4 months after the operation.
Development of a pulmonary arterial aneurysm can be caused by infection, a congenital cardiovascular anomaly, trauma, pulmonary hypertension, or vasculitis (1). We initially thought that the pulmonary arterial aneurysms of our case were caused by Behçet's disease. However, the diagnostic criteria of the International Study Group for Behçet's disease (5) state that recurrent oral ulceration must be present and accompanied by at least two of the following symptoms: recurrent genital ulceration, an eye lesion (such as uveitis or retinal vasculitis), a skin lesion, or a positive skin pathogen test. Our patient had none of these symptoms; Behçet's disease was therefore excluded.
Infection is the major cause of pulmonary artery aneurysms (1). The major pathogens are pyogenic microorganisms, including Staphylococcus and Streptococcus species. However, fungi, including Candida albicans and Aspergillus, have been rarely reported to be involved (6, 7, 8, 9). An infected pulmonary artery aneurysm can develop from 1) a hematogenous spread of an intraluminal septic thromboembolus into a vessel wall; 2) direct infection of the vessel from a focus of suppurating pulmonary infection; 3) ischemic injury to the pulmonary arterial wall caused by infection of the vasa vasorum; or 4) direct infectious inoculation of the vessel wall at the time of vascular trauma (1, 6). Of these mechanisms, the first (Candida endocarditis) may 208have caused the pulmonary aneurysms in our case.
Although pulmonary angiography is the diagnostic golden standard, CT and MRI have become important alternatives in diagnosis of pulmonary aneurysms. Both contrast-enhanced CT and MR imaging can clearly reveal a hyperenhancing nodule connected to a pulmonary vessel. If the density of the enhanced nodule is identical to that of the enhanced vessels, this is diagnostic for a pulmonary aneurysm (1). A few cases of pulmonary arterial mycotic aneurysms associated with Candida endocarditis have been reported (10, 11). However, to our knowledge, no prior report of vegetation on the tricuspid valve detected by chest CT scan has appeared.
Usually, a mycotic pulmonary artery aneurysm is managed surgically. Alternatives include aneurysmectomy, lobectomy, aneurysmorrhaphy, or banding. However, conservative management is chosen when no evidence of acute hemoptysis or other emergent symptoms are apparent, or when a patient is not a surgical candidate. Alternative nonsurgical therapeutic procedures, including transcatheter embolization of the aneurysm using steel coils or detachable balloons, have been reported to be safe and effective when used to prevent rupture of an aneurysm (1).
In conclusion, we present a case of a mycotic pulmonary arterial aneurysm associated with fungal endocarditis caused by Candida albicans. The case emphasizes that when pulmonary artery aneurysm is detected without evident pneumonia, it is important to consider a diagnosis of mycotic pulmonary aneurysm with observation of the heart. Also, we report the clinical course of a patient with a mycotic PAA and septic pulmonary emboli, who was treated conservatively.
Figures and Tables
Fig. 1
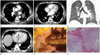
A 53-year-old male patient diagnosed with mycotic pulmonary artery aneurysm associated with Candida endocarditis.
A. An axial CT scan reveals an aneurysm with thrombus in the left inferior pulmonary artery (arrow).
B. An axial scan taken a few centimeters below the level of scan (A) reveals an aneurysm of the right posterior basal segmental pulmonary artery (arrow).
C. A coronal reformatted CT image at lung window setting shows no adjacent pneumonic consolidation or cavitary nodules in both lungs.
D. Two weeks after follow-up CT reveals a low attenuated mass (arrow) at the right atrioventricular septum, which is suspected to be thrombus or vegetation.
E. The surgical field contains large vegetations attached to the anterior (A) and septal (S) leaflets of the tricuspid valve.
F. A photomicrograph showing abundant fungal hyphae in vegetation removed from the tricuspid valve, consistent with the presence of Candida species (hematoxylin-eosin staining; × 400).
References
1. Kim HS, Oh YW, Noh HJ, Lee KY, Kang EY, Lee SY. Mycotic pulmonary artery aneurysm as an unusual complication of thoracic actinomycosis. Korean J Radiol. 2004; 5:68–71.
2. Müller KA, Zürn CS, Patrik H, Heuschmid M, Hetzel J, Henning A, et al. Massive haemoptysis in an intravenous drug user with infective tricuspid valve endocarditis. BMJ Case Rep. 2010; 2010.
3. Navarro C, Dickinson PC, Kondlapoodi P, Hagstrom JW. Mycotic aneurysms of the pulmonary arteries in intravenous drug addicts. Report of three cases and review of the literature. Am J Med. 1984; 76:1124–1113.
4. Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010; 121:1141–1152.
5. International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990; 335:1078–1080.
6. Lee WK, Mossop PJ, Little AF, Fitt GJ, Vrazas JI, Hoang JK, et al. Infected (mycotic) aneurysms: spectrum of imaging appearances and management. Radiographics. 2008; 28:1853–1868.
7. Sever M, Verstovsek S, Erasmus J Jr, Mattiuzzi GN. Mycotic pulmonary artery aneurysm due to Aspergillus infection in a patient with leukemia: case report and review of the literature. Leuk Res. 2010; 34:e133–e136.
8. Choyke PL, Edmonds PR, Markowitz RI, Kleinman CS, Laks H. Mycotic pulmonary artery aneurysm: complication of Aspergillus endocarditis. AJR Am J Roentgenol. 1982; 138:1172–1175.
9. Talwar S, Sharma R, Das B, Bhan A, Ray R, Saxena A, et al. Multiple fungal mycotic pulmonary artery aneurysms in an infant. Indian Heart J. 2000; 52:343–345.
10. Roush K, Scala-Barnett DM, Donabedian H, Freimer EH. Rupture of a pulmonary artery mycotic aneurysm associated with candidal endocarditis. Am J Med. 1988; 84:142–144.
11. Wijesekera NT, Sheppard MN, Mullen MJ. Candida endocarditis with mycotic pulmonary emboli following re-do Rastelli operation. Heart. 2004; 90:e34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download