Abstract
Purpose
To investigate the clinical significance of a new hypermetabolic lesion detected by fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) as well as the utility of second-look examinations to evaluate loco-regional recurrence of breast cancer.
Materials and Methods
Our database revealed 922 breast cancer patients who underwent surgery from January 2008 to July 2011. We included 20 patients with negative findings on routine follow-up but with new hypermetabolic lesions on FDG-PET/CT. All underwent second-look examination [breast ultrasound (US) = 14, chest CT scan = 6]. A total of 17 cases were pathologically verified and 3 were diagnosed with follow-up imaging.
Results
The locations were in the axillae (n = 7), breast (n = 6), chest wall (n = 3), cervical/supraclavicular (n = 3), and internal mammary (n = 1). Of the 20 hypermetabolic lesions, 10 were pathologically confirmed malignancies. Of the 14 patients who had undergone US, 7 had suspicious findings and 5 were confirmed as malignancies. Of a total of 6 patients who had undergone CT scans, 5 had suspicious findings and all turned out to be malignancies. The positive predictive value of the second-look examination was 83.3% (10/12).
Figures and Tables
 | Fig. 1A 30-year-old woman who had undergone left modified radical mastectomy for mucinous carcinoma presented with a lesion suspicious for malignancy on a 3-year follow-up fluorodeoxyglucose positron emission tomography (FDG-PET)/CT scan and confirmed as local recurrences on pathologic examination.
A. FDG-PET/CT scan shows multifocal hypermetabolic nodular lesions in left axilla, pectoralis muscle, shoulder, and brachialis muscle (arrows).
B. Second-look ultrasound shows multiple partially indistinct isoechoic masses (arrow) at the corresponding areas and these are considered to be BI-RADS category 5.
|
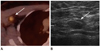 | Fig. 2A 54-year-old woman who had undergone left modified radical mastectomy presented with a lesion suspicious for malignancy on a 4.5-year follow-up fluorodeoxyglucose positron emission tomography (FDG-PET)/CT scan but confirmed as benign reactive hyperplasia on pathologic specimen.
A. FDG-PET/CT scan shows a newly developed hypermetabolic lesion in the right axilla (arrow) which is suspicious for metastatic lymphadenopathy.
B. Second-look ultrasound shows a hypoechoic lymph node (arrow) in the right axilla. It shows eccentric cortical thickening, and so is considered to be a malignant lymph node (BI-RADS category 4a).
|
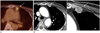 | Fig. 3A 67-year-old woman who underwent left modified radical mastectomy presented with a lesion concerning for malignancy on a 3 year 2 months follow-up fluorodeoxyglucose positron emission tomography (FDG-PET)/CT scan and confirmed to be a malignant lymph node on final pathologic examination.
A. FDG-PET/CT scan shows a focal hypermetabolic nodule (arrow) in the left internal mammary area.
B. Second-look chest CT scan shows a 0.5-cm enhancing nodular lesion (arrow) in the left internal mammary area. This is very small but not seen on previous imaging so considered to be a malignant lesion (BI-RADS category 4c).
|
 | Fig. 4Hypermetabolic lesions detected by fluorodeoxyglucose positron emission tomography/CT correlated with second-look imaging and pathologic findings. The overall sensitivity, specificity, positive predictive values, and negative predictive values of second-look examination are 100% (10/10), 80% (8/10), 83.3% (10/12), and 100% (8/8). |
References
1. Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2008. Bethesda, MD: National Cancer Institute;cited 2012 Sep. 7th. Available from: http://seer.cancer.gov/csr/1975_2008/.
2. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013; 45:1–14.
3. Moran MS, Haffty BG. Local-regional breast cancer recurrence: prognostic groups based on patterns of failure. Breast J. 2002; 8:81–87.
4. Cardoso F, Fallowfield L, Costa A, Castiglione M, Senkus E. ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011; 22:Suppl 6. vi25–vi30.
5. Cady B. Dilemmas in breast disease. Breast J. 1995; 1:121–124.
6. Eubank WB, Mankoff DA, Schmiedl UP, Winter TC 3rd, Fisher ER, Olshen AB, et al. Imaging of oncologic patients: benefit of combined CT and FDG PET in the diagnosis of malignancy. AJR Am J Roentgenol. 1998; 171:1103–1110.
7. Rieber A, Schirrmeister H, Gabelmann A, Nuessle K, Reske S, Kreienberg R, et al. Pre-operative staging of invasive breast cancer with MR mammography and/or PET: boon or bunk? Br J Radiol. 2002; 75:789–798.
8. Suárez M, Peréz-Castejón MJ, Jiménez A, Domper M, Ruiz G, Montz R, et al. Early diagnosis of recurrent breast cancer with FDG-PET in patients with progressive elevation of serum tumor markers. Q J Nucl Med. 2002; 46:113–121.
9. Gallowitsch HJ, Kresnik E, Gasser J, Kumnig G, Igerc I, Mikosch P, et al. F-18 fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the follow-up of patients with breast carcinoma: a comparison to conventional imaging. Invest Radiol. 2003; 38:250–256.
10. Aukema TS, Rutgers EJ, Vogel WV, Teertstra HJ, Oldenburg HS, Vrancken Peeters MT, et al. The role of FDG PET/CT in patients with locoregional breast cancer recurrence: a comparison to conventional imaging techniques. Eur J Surg Oncol. 2010; 36:387–392.
11. Grahek D, Montravers F, Kerrou K, Aide N, Lotz JP, Talbot JN. [18F]FDG in recurrent breast cancer: diagnostic performances, clinical impact and relevance of induced changes in management. Eur J Nucl Med Mol Imaging. 2004; 31:179–188.
12. Radan L, Ben-Haim S, Bar-Shalom R, Guralnik L, Israel O. The role of FDG-PET/CT in suspected recurrence of breast cancer. Cancer. 2006; 107:2545–2551.
13. Veit-Haibach P, Antoch G, Beyer T, Stergar H, Schleucher R, Hauth EA, et al. FDG-PET/CT in restaging of patients with recurrent breast cancer: possible impact on staging and therapy. Br J Radiol. 2007; 80:508–515.
14. Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998; 39:431–435.
15. Adler LP, Faulhaber PF, Schnur KC, Al-Kasi NL, Shenk RR. Axillary lymph node metastases: screening with [F-18]2-deoxy-2-fluoro-D-glucose (FDG) PET. Radiology. 1997; 203:323–327.
16. Utech CI, Young CS, Winter PF. Prospective evaluation of fluorine-18 fluorodeoxyclucose positron emission tomography in breast cancer for staging of the axilla related to surgery and immunocytochemistry. Eur J Nucl Med. 1996; 23:1588–1593.
17. Avril N, Dose J, Jänicke F, Bense S, Ziegler S, Laubenbacher C, et al. Metabolic characterization of breast tumors with positron emission tomography using F-18 fluorodeoxyglucose. J Clin Oncol. 1996; 14:1848–1857.
18. Isasi CR, Moadel RM, Blaufox MD. A meta-analysis of FDG-PET for the evaluation of breast cancer recurrence and metastases. Breast Cancer Res Treat. 2005; 90:105–112.
19. Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998; 16:3375–3379.
20. Murakami R, Kumita S, Yoshida T, Ishihara K, Kiriyama T, Hakozaki K, et al. FDG-PET/CT in the diagnosis of recurrent breast cancer. Acta Radiol. 2012; 53:12–16.
21. National Institute for Health and Clinical Excellence. National Collaborating Centre for Cancer. Early and locally advanced breast cancer: diagnosis and treatment. London: National Institute for Health and Clinical Excellence;2009.
22. Belli P, Costantini M, Romani M, Marano P, Pastore G. Magnetic resonance imaging in breast cancer recurrence. Breast Cancer Res Treat. 2002; 73:223–235.
23. Goerres GW, Michel SC, Fehr MK, Kaim AH, Steinert HC, Seifert B, et al. Follow-up of women with breast cancer: comparison between MRI and FDG PET. Eur Radiol. 2003; 13:1635–1644.
24. Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010; 375:563–571.
25. Viehweg P, Heinig A, Lampe D, Buchmann J, Heywang-Köbrunner SH. Retrospective analysis for evaluation of the value of contrast-enhanced MRI in patients treated with breast conservative therapy. MAGMA. 1998; 7:141–152.
26. Bleicher RJ, Ciocca RM, Egleston BL, Sesa L, Evers K, Sigurdson ER, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009; 209:180–187. quiz 294-295.
27. Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin. 2009; 59:290–302.
28. Nori J, Vanzi E, Bazzocchi M, Bufalini FN, Distante V, Branconi F, et al. Role of axillary ultrasound examination in the selection of breast cancer patients for sentinel node biopsy. Am J Surg. 2007; 193:16–20.
29. Lee MC, Eatrides J, Chau A, Han G, Kiluk JV, Khakpour N, et al. Consequences of axillary ultrasound in patients with T2 or greater invasive breast cancers. Ann Surg Oncol. 2011; 18:72–77.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download