Abstract
Purpose
To evaluate the clinical effectiveness of an intermittent computed tomography (CT) fluoroscopy-guided radiofrequency (RF) ablation of hepatocellular carcinoma located in the liver dome.
Materials and Methods
Between 2005 and 2010 23 patients with hepatocellular carcinoma (HCC) nodules located in the liver dome underwent an intermittent CT fluoroscopy-guided RF ablation. The primary endpoint was the local tumor progression. Procedure-related complications occurred in 3 of 23 patients. To evaluate the prognostic factors for the local tumor progression, univariate and multivariate analyses were performed using the Cox proportional hazards model. The chi-squared test was performed to evaluate the association of access route and procedure-related complication. The study was approved by the Institutional Review Board of our hospital.
Results
The Tumor sizes ranged between 1.0 and 2.9 cm. An initial complete ablation was achieved in all patients. The median follow-up period was 31 months and the major complication rate was 4.3%. The cumulative rate of local tumor progression at 3 years was 20%. The univariate analysis revealed that only serum total bilirubin level (p = 0.048) and prior chemoembolization were statistically significant (p = 0.044), but there was no independently significant prognostic factor on multivariate analysis. Procedure-related complications occurred in 3 of 23 patients.
Percutaneous ultrasonography (US) guided radiofrequency (RF) ablation of liver tumors is a widely used technique (1-4). However, many hepatocellular carcinoma (HCC) nodules located in the liver dome are poorly visualized on the US scan (5-7). A conventional CT guided RF ablation can overcome this limitation, but has a significant disadvantage because the real-time monitoring is not possible (8, 9).
RF ablation under real-time CT fluoroscopy guidance can by reconstruction and display of CT images in real-time overcome this limitation (10). This possibility provides the operator with an immediate feedback of images during the procedure, even if the nodules are not visualized on the US (11, 12). Unfortunately, the radiation hazards caused by this technique can be substantial to both the patients and the operator (13-16). If an intermittent CT fluoroscopy guided RF ablation is performed using the quick check method the radiation dose to the operators and the patients can be reduced (16-18). The aim of the present study was to evaluate whether intermittent CT fluoroscopy-guided RF ablation can be a clinically effective treatment for HCC nodules located in the liver dome.
The study was a retrospective analysis based on a prospective database and was approved by the Institutional Review Board of our hospital. An informed consent for interventional procedures was obtained from all patients before.
The primary endpoint was defined as local tumor progression. The secondary endpoints included initial complete ablation, major and minor complications and overall survival.
All CT fluoroscopy guided RF ablations were blinded performed by a single interventional radiologist with seven years of experience in the ablation of liver tumors at study onset.
Twenty-three consecutive subjects with hepatocellular carcinoma located in the liver dome were included between December 2005 and December 2010. Because the HCC were not clearly visible on US, all patients underwent a CT fluoroscopy-guided RF ablation for HCC. HCC nodules located within 2 cm from the diaphragm were classified as liver dome nodules. CT fluoroscopy guided RF ablation was performed for primary or recurrent HCC nodules following transarterial chemoembolization (TACE).
The inclusion criteria for percutaneous RF ablation were as follows: a single tumor of 4 cm in longest dimension or smaller; multinodular tumors (≤ 3) with each tumor ≤ 3 cm in the longest dimension, Child-Pugh class A or B; no portal vein thrombosis or extrahepatic metastasis; prothrombin time ratio greater than 50% and platelet count greater than 50000/mm3 (50 × 109/L).
CT fluoroscopy guided RF ablation was not performed if a patient was not cooperative.
The diagnosis of HCC was based on the typical imaging features which are an arterial enhancement followed by delayed washout at the dynamic contrast-enhanced CT or MRI (1). No patient underwent a percutaneous biopsy to diagnose HCC.
CT fluoroscopic images were acquired during the intermittent scanning with a machine (LightSpeed CT/i equipped with SmartStep; GE Medical Systems, Milwaukee, WI, USA), which was controlled by an integrated foot switch and hand-held controller. The radiation exposure time was 0.8 or 1 second, and the reconstruction duration for individual CT fluoroscopic spot images was 5 seconds (17, 18). Gantry tilt was possible up to 30 degree. The reconstruction of an image was performed with a 256 × 256 matrix, with images displayed on a 768 × 768 matrix.
The anticipated path and depth of the target were determined on the preliminary CT scans by using the electronic calipers on the technologist's console. The anticipated skin entry site was prepared with providone iodine solution (Betadine, Purdue Frederick, Norwalk, CT, USA) and draped in the routine sterile fashion. After local anesthetization in the anticipated puncture site, RF ablation was performed under conscious sedation using a combination of intravenous fentanyl citrate (Myung-moon, Seoul, Korea) and midazolam (Bukwang, Seoul, Korea).
After the site was prepared and draped, targeting was with the quick-check technique which is the most widely used intermittent CT fluoroscopy technique actually (17, 18). After the abdominal wall has been punctured, the table was moved to align the RF electrode with the imaging plane using the laser marker. An internally cooled single electrode with 3 cm exposed tip (Cool tip; Radionics, Burlington, MA, USA) or multi-tined electrodes with 3.5 cm exposed tip (Le Veen Needle electrode; Boston Scientific, Watertown, MA, USA) were used. Then single CT fluoroscopic spot images were acquired to check the electrode location and to confirm the appropriate alignment. The gantry was tilted until the lung parenchyma was not included in the imaginary trajectory on the preliminary scan. If this was not possible, a trans-pulmonary approach was used. Once the single CT fluoroscopic spot image helped to confirm that the electrode was at the appropriate position and trajectory, the electrode was advanced to the target (17, 18). While advancing the electrode, intermittent fluoroscopic spot images were acquired to confirm the electrode locations until the electrode tip was positioned in the target. The tube potentials were 140 kVp, the tube currents were 50 mA and the section thickness was 5 mm.
If possible, 5-10 mm of ablative margin around the index tumor if were secured. An attempt was also done to limit the number of electrodes passes through the peritoneum to a single insertion. If an additional ablation was acquired, the needle position within the tumor was relocated by pushing it back into the superficial liver tissue along its major axis, changing the angle and then inserting the needle into the target without a complete withdrawal of the electrode out of the peritoneum.
Enhanced dynamic CT scans were performed immediately after the RF procedure to evaluate the possible residual viable tumors. The total procedure time (from the injection of local anesthetics to the time of needle withdrawal) and the total fluoroscopic time (in seconds) were reported. The number of peritoneal punctures and the number of liver parenchymal puncture tracts were also recorded. As a measure of therapeutic responses, initial complete ablations, complications, local tumor progression and the overall survival of patients following RF ablation were evaluated.
Technique effectiveness of CT fluoroscopy-guided RF ablation was defined as complete encompassment of index tumor by ablation zone on dynamic CT one month post procedure (19). Residual viable tumor was judged as present when an enhanced portion was seen within or around of the original mass on the one-month follow-up CT imaging. If no definite evidence of residual tumor was noted on the one-month follow-up CT, a 3-phase contrast-enhanced CT or dynamic MRI was performed at 3- or 4-month interval thereafter. Local tumor progression was judged as present when an enhanced portion was seen within or at the margin of the original mass at the first one-month or the thereafter follow-up dynamic CT or MRI scans. The CT or MR images were evaluated by two radiologists (K.M.Y. and C.H.J.) with 5 and 15 years of experience in abdominal radiology. Final decisions were reached by consensus. Major complication was defined as an event that leads to substantial morbidity or substantially lengthened hospital stay (19).
The cumulative local tumor progression rates were estimated by using the Kaplan-Meier estimation. To evaluate the possible prognostic factors of local tumor progression, univariate and multivariate analyses were performed using the Cox proportional hazard model. Parameters that proved to be significant in the univariate analysis were subsequently tested with the multivariate Cox proportional hazard model. The chi-squared test was performed to evaluate the association of access route and the occurrence of complication after the procedure.
Null hypotheses of no difference was rejected if p-values were less than 0.05. The SPSS software package (version 10.0; SPSS Inc., Chicago, IL, USA) was used for the statistical analysis.
Age of patients ranged between 56 and 78 years (mean age 64.9 years). All 23 patients had cirrhosis associated with either viral hepatitis B (16 patients), hepatitis C (five patients) or other unknown causes (two patients). Among the patients, 22 had Child-Pugh class A and one had class B disease. The tumor size ranged from 1.0 cm to 2.9 cm (median 1.7 cm). The follow-up periods ranged from 9 to 70 months (median 31 months). Prior to the performance of CT fluoroscopy guided RF ablation 17 out of the 23 patients underwent a TACE for the index tumors. Internally cooled single electrode or multi-tined electrodes were used in 17 respective in 6 patients. Other factors are described in Table 1.
The total procedural time per session ranged from 30 to 100 minutes (average time 58 minutes) and the CT fluoroscopic time per session ranged from 2 seconds to 52 seconds (average time 16 seconds). The number of peritoneal puncture ranged from one to three times (average time 1.2 times), while the number of needle tracts in the liver parenchyma per session ranged from 1 to 8 (average time 3.0). The ablation time ranged from 6 to 24 minutes and a roll-off was obtained in all patients.
No residual viable tumors were noted in all patients after one or two initial sessions of CT fluoroscopy guided RF ablation. One session of CT fluoroscopy guided RF ablation was performed in 21 patients, and in two patients were performed two sessions (Fig. 1). The technique effectiveness rate was 91%. Local tumor progression occurred in three of the 23 nodules. An additional TACE or RF ablation was performed in two patients to treat these recurrent tumors. The cumulative local tumor progression rates were 0% after 1 year, 12% after 2 years and 20% after 3 years (Fig. 2). Table 2 shows the results of univariate analysis for the prognostic factors for local recurrence. Only the level of serum total bilirubin level (> 1 mg/dL, p = 0.048) and prior TACE (p = 0.044) were statistically significant. In the univariate analysis no other factors were statistically significant. Multivariate analysis revealed no independent significant prognostic factors.
In three of the 23 patients procedure-related complications occurred, including minor pleural effusion in two patients, self-limited pneumothorax in one patient and an intraperitoneal hematoma in one patient. The latter was the only major complication and it was successfully treated with conservative management. The overall complication rate per patient was 13.0% (3/23) and the major complication rate was 4.3% (1/23). However, no major complication occurred under transabdominal approaches and three of the four complications occurred among the six patients who underwent a RF ablation with a transpulmonary approach. The chi-squared test revealed that the access route and the occurrence of complication were statistically associated (p = 0.040).
Fifteen patients died during the follow-up period and among them 10 patients died with progressive HCC. The 1-, 2-, 3-, 4-, and 5-year overall survival rates from the initial diagnosis of HCC were 96%, 91%, 82%, 68%, and 48%, respectively. The 1-, 2-, 3-, 4-, and 5-year overall survival rates from the performance of RF ablation for the HCC nodule were 83%, 63%, 58%, 42%, and 31%, respectively.
To perform an ultrasonographically guided RF ablation for the HCC nodules located in the liver dome is difficult in many cases because of their poor visibility or accessibility (2, 20). These tumors may become clearly visualized if an artificial ascites or pleural effusion is induced (20-22). However, inducing an artificial pleural effusion can be very difficult in cases of pleural adhesion (21, 22). Even after an artificial ascites or pleural effusion are induced successfully, the local tumor progression rates are reported as 20% or more, because it may still be difficult to localize the tumor accurately (20-23). Furthermore, because of the presence of diffusely scattered benign nodules in the background liver parenchyma many small tumors cannot be clearly discerned (24, 25).
Real-time guidance techniques other than US, such as the real time CT fluoroscopy, biplane fluoroscopy or 3-dimensional (3D) fluoroscopic navigation system using cone beam CT, can be potent guiding techniques also (14, 26, 27). However, biplane fluoroscopy guidance techniques or 3D fluoroscopic navigation system are useful only when the tumors are clearly visible on the fluoroscopy because of the iodized oil retention within the tumors (27). In addition, with real time CT fluoroscopy guidance technique the radiation hazard can be substantial to both the patient and the operator (14).
In contrast, an intermittent CT fluoroscopy guided RF ablation can be with a minimal risk of mistargeting or radiation hazard an effective alternative method for HCC regardless of the iodized oil retention (16). In addition, an easier localization process is the important advantage of CT fluoroscopy if compared with the conventional CT guidance technique. Also, an interactive process is possible between the operator and the patient (16).
In the present study an initial complete ablation was achieved in all cases, but the cumulative rate of local tumor progression at 20% after 3 years. This is similar to the results from previous studies where conventional CT guidance or biplane fluoroscopy guidance techniques were used (20, 26, 28). Such a suboptimal result may be partially attributed to the difficulties to accurate localize the respiratory movements of patients during the procedure. The radiation dose needs to be in acceptable ranges for both the patient and the personnel if the intermittent-mode CT fluoroscopy-guided RF ablation was to be justified. The average CT fluoroscopic times in the present study were similar to those reported for other intra-abdominal procedures such as biopsy or drainage procedures using the intermittent-mode CT fluoroscopy guidance (17, 18).
In the present study, a transabdominal approach was preferred with an intention to minimize possible pleural and lung parenchymal injuries or a metastatic tumor seeding in the thorax (29). A frequent incidence of pneumothorax or pleural effusion has been reported for CT guided RF ablation using a transpulmonary approach (29-31). Among the 17 patients treated with a transdominal approach only one case of minor pleural effusion was encountered as a procedure-related complication which is in accordance with other study results. In contrast, if the transpulmonary approach was used, the complication rate was much higher. So, in the authors' opinion, transpulmonary approaches should be rather avoided if possible to reduce the complication rates.
The limitations of the present study are as follows:
First, the study was a single arm retrospective analysis. Further studies by other investigators will be needed for generalization. Prospective controlled studies will provide a strong conclusion on the clinical effectiveness of the intermittent CT fluoroscopy guided RF ablation.
Second, prior TACE might have a significant confounding effect on the clinical effectiveness of the CT fluoroscopy guided RF ablation. Conversely, the present study strongly suggests a prior treatment with TACE may reduce the local tumor progression significantly (32).
Third, the local tumor progression rate for HCC nodules in liver dome was not reduced even with a higher radiation exposure to both the patient and the personnel than suggested by the conventional CT guidance or the biplane fluoroscopy guidance.
Finally, the number of patients was too small to reveal potentially significant prognostic factors for local tumor progression in the multivariate analysis.
In conclusion, the intermittent CT fluoroscopy-guided RF ablation can be performed safely and effectively for the HCCs located in the liver dome which are not clearly visible on US.
Figures and Tables
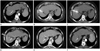 | Fig. 1A 62-year-old woman with hepatocellular carcinoma located in the right hepatic dome.
A. Arterial phase of the contrast-enhanced computed tomography (CT) scan shows a 1.6 cm sized hypervascular nodule with spotty iodized oil uptake in liver (arrow). Delayed phase CT scan (not shown here) reveals washout of contrast media, confirming the radiologic diagnosis of hepatocellular carcinoma (HCC).
B. Intermittent CT fluoroscopic spot images are taken to confirm that the electrode is at the appropriate position and trajectory until the electrode tip is positioned in the target.
C. On an unenhanced CT scan obtained during the procedure of radiofrequency (RF) ablation under CT fluoroscopy guided transhepatic approach, the tins of a multitined expandable electrode are deployed within the tumor. The duration of application of RF energy was 13 minutes.
D. Portal phase of the contrast-enhanced CT scan obtained immediately after the RF ablation shows a 2.4 cm diameter ablated zone (arrow). The RF needle was not completely retracted before the operator convinced that the tumor has been sufficiently ablated.
E. Arterial (not shown here) and portal phase of the contrast-enhanced CT scan obtained one month after RF ablation shows no definite evidence of residual or recurred HCC nodule (arrow).
F. Arterial phase of the contrast-enhanced CT scan obtained 68 months after RF ablation shows no definite evidence of residual or recurred HCC nodules (arrow). Note that the extent of the ablated area has been much reduced than before.
|
 | Fig. 2A diagram of the cumulative local tumor progression rate of hepatocellular carcinoma after RF ablation under CT fluoroscopy. Note that the cumulative 3-year local tumor progression rate was 20%. |
References
1. Bruix J, Sherman M. Practice Guidelines Committee. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.
2. Rhim H, Lim HK, Kim YS, Choi D, Lee WJ. Radiofrequency ablation of hepatic tumors: lessons learned from 3000 procedures. J Gastroenterol Hepatol. 2008; 23:1492–1500.
3. Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005; 103:1201–1209.
4. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008; 47:82–89.
5. Yang B, Zhang B, Xu Y, Wang W, Shen Y, Zhang A, et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol. 1997; 123:357–360.
6. Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995; 22:432–438.
7. Izzo F, Cremona F, Ruffolo F, Palaia R, Parisi V, Curley SA. Outcome of 67 patients with hepatocellular cancer detected during screening of 1125 patients with chronic hepatitis. Ann Surg. 1998; 227:513–518.
8. Toyoda M, Kakizaki S, Horiuchi K, Katakai K, Sohara N, Sato K, et al. Computed tomography-guided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol. 2006; 12:608–611.
9. Park BJ, Byun JH, Jin YH, Won HJ, Shin YM, Kim KW, et al. CT-guided radiofrequency ablation for hepatocellular carcinomas that were undetectable at US: therapeutic effectiveness and safety. J Vasc Interv Radiol. 2009; 20:490–499.
10. Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, et al. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002; 13:1225–1232.
11. Yamakado K, Nakatsuka A, Takaki H, Yokoi H, Usui M, Sakurai H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology. 2008; 247:260–266.
12. Katada K, Kato R, Anno H, Ogura Y, Koga S, Ida Y, et al. Guidance with real-time CT fluoroscopy: early clinical experience. Radiology. 1996; 200:851–856.
13. Kato R, Katada K, Anno H, Suzuki S, Ida Y, Koga S. Radiation dosimetry at CT fluoroscopy: physician's hand dose and development of needle holders. Radiology. 1996; 201:576–578.
14. Nawfel RD, Judy PF, Silverman SG, Hooton S, Tuncali K, Adams DF. Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology. 2000; 216:180–184.
15. Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994; 5:71–84.
16. Silverman SG, Tuncali K, Adams DF, Nawfel RD, Zou KH, Judy PF. CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology. 1999; 212:673–681.
17. Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT. CT fluoroscopy--guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001; 220:161–167.
18. Carlson SK, Felmlee JP, Bender CE, Ehman RL, Classic KL, Hoskin TL, et al. CT fluoroscopy-guided biopsy of the lung or upper abdomen with a breath-hold monitoring and feedback system: a prospective randomized controlled clinical trial. Radiology. 2005; 237:701–708.
19. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:7 Suppl. S377–S390.
20. Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR Am J Roentgenol. 2008; 190:91–98.
21. Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, et al. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004; 183:583–588.
22. Iwai S, Sakaguchi H, Fujii H, Kobayashi S, Morikawa H, Enomoto M, et al. Benefits of artificially induced pleural effusion and/or ascites for percutaneous radiofrequency ablation of hepatocellular carcinoma located on the liver surface and in the hepatic dome. Hepatogastroenterology. 2012; 59:546–550.
23. Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009; 10:34–42.
24. Fukunaga T, Kudo M, Tochio H, Okabe Y, Orino A. Natural course of small nodular lesions with intranodular preserved portal supply in cirrhotic liver. Oncology. 2007; 72:Suppl 1. 24–29.
25. Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009; 44:Suppl 19. 112–118.
26. Lee MW, Kim YJ, Park SW, Yu NC, Choe WH, Kwon SY, et al. Biplane fluoroscopy-guided radiofrequency ablation combined with chemoembolisation for hepatocellular carcinoma: initial experience. Br J Radiol. 2011; 84:691–697.
27. Morimoto M, Numata K, Kondo M, Nozaki A, Hamaguchi S, Takebayashi S, et al. C-arm cone beam CT for hepatic tumor ablation under real-time 3D imaging. AJR Am J Roentgenol. 2010; 194:W452–W454.
28. Kim YK, Kim CS, Lee JM, Chung GH, Chon SB. Efficacy and safety of radiofrequency ablation of hepatocellular carcinoma in the hepatic dome with the CT-guided extrathoracic transhepatic approach. Eur J Radiol. 2006; 60:100–107.
29. Wang ZY, Sun WB, Li MY, Zhang XX, Ding XM. Percutaneous extrapulmonary radiofrequency ablation for tumors in the hepatic dome. Hepatogastroenterology. 2008; 55:1164–1166.
30. Shibata T, Shibata T, Maetani Y, Kubo T, Itoh K, Togashi K, et al. Transthoracic percutaneous radiofrequency ablation for liver tumors in the hepatic dome. J Vasc Interv Radiol. 2004; 15:1323–1327.
31. Miura H, Yamagami T, Terayama K, Yoshimatsu R, Matsumoto T, Nishimura T. Pneumothorax induced by radiofrequency ablation for hepatocellular carcinoma beneath the diaphragm under real-time computed tomography-fluoroscopic guidance. Acta Radiol. 2010; 51:613–618.
32. Lee MW, Kim YJ, Park SW, Hwang JH, Jung SI, Jeon HJ, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br J Radiol. 2009; 82:908–915.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download