Abstract
Purpose
We correlated cerebrovascular reserve in unilateral high grade carotid artery stenosis or occlusion with a type of collateral circulation using acetazolamide-challenged perfusion CT (ACZ-PCT).
Materials and Methods
Among the patients who underwent ACZ-PCT in our institution, we retrospectively selected the patients with unilateral high grade internal carotid artery or middle cerebral artery stenosis (> 70%) or occlusion; we verified the types of their dominant collateral circulation by digital subtraction angiography or 3T MR-angiography; first, the primary collaterals flow through the circle of Willis; second, the secondary collaterals that flow through the opthalmic artery, the basal artery or other external carotid artery. Using ACZ-PCT, we measured the difference in percentage change of cerebral blood flow of the stenotic hemisphere against contralateral normal hemisphere and compared cerebrovascular reserves of lesional hemisphere, according to the type of collaterals.
Results
A total of 28 patients were included. The percentage changes of cerebral blood flow were significantly lower in the stenotic hemisphere than the contralateral hemisphere (14.34 ± 36.43% and 34.53 ± 47.82%, p < 0.001), and in the hemisphere predominantly supplied by secondary collaterals than primary (7.03 ± 32.71% and 24.37 ± 42.03%, p < 0.05), respectively.
Figures and Tables
Fig. 1
Locations of regions of interest (ROI) in cerebral perfusion CT.
A, B. Diffusion weighted image (A) and apparent diffusion coefficient map (B) show acute infarction at left basal ganglia in a 50-year male patient.
C. Four ROIs in both middle cerebral artery (MCA) territories of perfusion CT before and after acetazolamide infusion are demonstrated at the level of basal ganglia.
D. This is a representative arterial input and venous outflow time-attenuation curves at left MCA cortical flow territory. This time-attenuation curve is then used to calculate the perfusion CT parameters.
E-H. Examples of cerebral blood volume (CBV), cerebral blood flow (CBF), mean transit time (MTT), and time to peak enhancement (TTP) on perfusion CT before acetazolamide infusion at the level of basal ganglia (right to left).
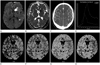
Fig. 2
Percentage change of cerebral blood flow (CBF) in patient with left proximal middle cerebral artery occlusion with secondary collaterals. A 55-year-old woman without evidence of ischemic symptom.
A. There is no definable abnormal sign on the initial brain MRI T2 weighted image.
B, C. Left middle cerebral artery is occluded just distal to distal internal cerebral artery bifurcation on time of flight MR angiography (B) and digital subtraction angiography (DSA) (C).
D. Secondary collaterals from ipsilateral anterior cerebral artery and external carotid artery are noted on DSA.
E. Relative preservation of CBF is shown in left frontotemporal cortex on baseline perfusion study, the value are 52.48 mL/100 g/min and 50.92 mL/100 g/min in occlusive and mirrored hemisphere, respectively.
F. But there is paradoxical response in stenotic area on post-acetazolamide study. Percentage change of CBF is -8.44%, suggesting significant decreased cerebrovascular reserve as compared with 21.70% in contralateral cortex.
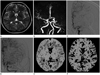
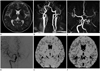
Fig. 3
Percentage change of cerebral blood flow (CBF) in patient with left proximal internal cerebral artery (ICA) occlusion with primary collaterals. A 71-year-old woman without ischemic symptom.
A. There is no demonstrable abnormal finding on the initial brain MRI T2 weighted image.
B, C. Total occlusion of left proximal ICA is demonstrated on contrast-enhanced MR angiography (MRA) (B). However, left middle cerebral artery (MCA) is visualized on her time of flight MRA through anterior communicating artery (C).
D. Ipsilateral anterior cerebral artery, MCA and their cortical branches distal to occluded left ICA are visualized on digital subtraction angiography, suggesting primary collaterals.
E, F. Cerebral blood flow maps of baseline (E) and post-acetazolamide (F) cerebral perfusion study show relative preserved cerebrovascular reserve in left frontotemporal cortex. The percentage change of CBF is 32.88% and 35.87% is measured in mirrored hemisphere.
References
1. Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Cloft HJ, Chimowitz MI. Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Investigators. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. 2011; 31:1293–1301.
2. Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Connolly ES. Radiographic and clinical predictors of hemodynamic insufficiency in patients with athero-occlusive disease. J Stroke Cerebrovasc Dis. 2008; 17:340–343.
3. Gupta A, Nair S, Schweitzer AD, Kishore S, Johnson CE, Comunale JP, et al. Neuroimaging of cerebrovascular disease in the aging brain. Aging Dis. 2012; 3:414–425.
4. Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke. 2012; 43:2884–2891.
5. Romero JR, Pikula A, Nguyen TN, Nien YL, Norbash A, Babikian VL. Cerebral collateral circulation in carotid artery disease. Curr Cardiol Rev. 2009; 5:279–288.
6. Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke. 1999; 30:593–598.
7. Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. The pattern of leptomeningeal collaterals on CT angiography is a strong predictor of long-term functional outcome in stroke patients with large vessel intracranial occlusion. Stroke. 2010; 41:2316–2322.
8. Drakou AA, Koutsiaris AG, Tachmitzi SV, Roussas N, Tsironi E, Giannoukas AD. The importance of ophthalmic artery hemodynamics in patients with atheromatous carotid artery disease. Int Angiol. 2011; 30:547–554.
9. van Everdingen KJ, Visser GH, Klijn CJ, Kappelle LJ, van der Grond J. Role of collateral flow on cerebral hemodynamics in patients with unilateral internal carotid artery occlusion. Ann Neurol. 1998; 44:167–176.
10. Cheng XQ, Tian JM, Zuo CJ, Liu J, Zhang Q, Lu GM. Quantitative perfusion computed tomography measurements of cerebral hemodynamics: correlation with digital subtraction angiography identified primary and secondary cerebral collaterals in internal carotid artery occlusive disease. Eur J Radiol. 2012; 81:1224–1230.
11. Kawashima M, Noguchi T, Yakushiji Y, Takase Y, Matsushima T. Leptomeningeal collateral and cerebral hemodynamics in patients with ICA and MCA steno-occlusion. Neurol Res. 2011; 33:307–313.
12. Hofmeijer J, Klijn CJ, Kappelle LJ, Van Huffelen AC, Van Gijn J. Collateral circulation via the ophthalmic artery or leptomeningeal vessels is associated with impaired cerebral vasoreactivity in patients with symptomatic carotid artery occlusion. Cerebrovasc Dis. 2002; 14:22–26.
13. Yamauchi H, Kudoh T, Sugimoto K, Takahashi M, Kishibe Y, Okazawa H. Pattern of collaterals, type of infarcts, and haemodynamic impairment in carotid artery occlusion. J Neurol Neurosurg Psychiatry. 2004; 75:1697–1701.
14. Kaku Y, Iihara K, Nakajima N, Kataoka H, Fukushima K, Iida H, et al. The leptomeningeal ivy sign on fluid-attenuated inversion recovery images in moyamoya disease: positron emission tomography study. Cerebrovasc Dis. 2013; 36:19–25.
15. Kawashima M, Noguchi T, Takase Y, Ootsuka T, Kido N, Matsushima T. Unilateral hemispheric proliferation of ivy sign on fluid-attenuated inversion recovery images in moyamoya disease correlates highly with ipsilateral hemispheric decrease of cerebrovascular reserve. AJNR Am J Neuroradiol. 2009; 30:1709–1716.
16. Andaluz N, Choutka O, Vagal A, Strunk R, Zuccarello M. Patient selection for revascularization procedures in adult Moyamoya disease based on dynamic perfusion computerized tomography with acetazolamide challenge (PCTA). Neurosurg Rev. 2010; 33:225–232. discussion 232-233.
17. Mendelowitsch A, Taussky P, Rem JA, Gratzl O. Clinical outcome of standard extracranial-intracranial bypass surgery in patients with symptomatic atherosclerotic occlusion of the internal carotid artery. Acta Neurochir (Wien). 2004; 146:95–101.
18. Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol. 2009; 30:876–884.
19. Kang KH, Kim HS, Kim SY. Quantitative cerebrovascular reserve measured by acetazolamide-challenged dynamic CT perfusion in ischemic adult Moyamoya disease: initial experience with angiographic correlation. AJNR Am J Neuroradiol. 2008; 29:1487–1493.
20. Rim NJ, Kim HS, Shin YS, Kim SY. Which CT perfusion parameter best reflects cerebrovascular reserve?: correlation of acetazolamide-challenged CT perfusion with single-photon emission CT in Moyamoya patients. AJNR Am J Neuroradiol. 2008; 29:1658–1663.
21. Smith LM, Elkins JS, Dillon WP, Schaeffer S, Wintermark M. Perfusion-CT assessment of the cerebrovascular reserve: a revisit to the acetazolamide challenges. J Neuroradiol. 2008; 35:157–164.
22. Lui YW, Tang ER, Allmendinger AM, Spektor V. Evaluation of CT perfusion in the setting of cerebral ischemia: patterns and pitfalls. AJNR Am J Neuroradiol. 2010; 31:1552–1563.
23. Eicker SO, Turowski B, Heiroth HJ, Steiger HJ, Hänggi D. A comparative study of perfusion CT and 99m Tc-HMPAO SPECT measurement to assess cerebrovascular reserve capacity in patients with internal carotid artery occlusion. Eur J Med Res. 2011; 16:484–490.
24. Lell M, Fellner C, Baum U, Hothorn T, Steiner R, Lang W, et al. Evaluation of carotid artery stenosis with multisection CT and MR imaging: influence of imaging modality and postprocessing. AJNR Am J Neuroradiol. 2007; 28:104–110.
25. Liebeskind DS. Collateral circulation. Stroke. 2003; 34:2279–2284.
26. Ozsarlak O, Van Goethem JW, Maes M, Parizel PM. MR angiography of the intracranial vessels: technical aspects and clinical applications. Neuroradiology. 2004; 46:955–972.
27. McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012; 33:576–582.
28. Bokkers RP, Wessels FJ, van der Worp HB, Zwanenburg JJ, Mali WP, Hendrikse J. Vasodilatory capacity of the cerebral vasculature in patients with carotid artery stenosis. AJNR Am J Neuroradiol. 2011; 32:1030–1033.
29. Alexandrov AV, Nguyen HT, Rubiera M, Alexandrov AW, Zhao L, Heliopoulos I, et al. Prevalence and risk factors associated with reversed Robin Hood syndrome in acute ischemic stroke. Stroke. 2009; 40:2738–2742.
30. Valdueza JM, Draganski B, Hoffmann O, Dirnagl U, Einhäupl KM. Analysis of CO2 vasomotor reactivity and vessel diameter changes by simultaneous venous and arterial Doppler recordings. Stroke. 1999; 30:81–86.
31. Sharma VK, Tsivgoulis G, Ning C, Teoh HL, Bairaktaris C, Chong VF, et al. Role of multimodal evaluation of cerebral hemodynamics in selecting patients with symptomatic carotid or middle cerebral artery steno-occlusive disease for revascularization. J Vasc Interv Neurol. 2008; 1:96–101.
32. Chen A, Shyr MH, Chen TY, Lai HY, Lin CC, Yen PS. Dynamic CT perfusion imaging with acetazolamide challenge for evaluation of patients with unilateral cerebrovascular steno-occlusive disease. AJNR Am J Neuroradiol. 2006; 27:1876–1881.
33. Orrison WW Jr, Snyder KV, Hopkins LN, Roach CJ, Ringdahl EN, Nazir R, et al. Whole-brain dynamic CT angiography and perfusion imaging. Clin Radiol. 2011; 66:566–574.
34. Yonas H, Darby JM, Marks EC, Durham SR, Maxwell C. CBF measured by Xe-CT: approach to analysis and normal values. J Cereb Blood Flow Metab. 1991; 11:716–725.
35. Eskey CJ, Sanelli PC. Perfusion imaging of cerebrovascular reserve. Neuroimaging Clin N Am. 2005; 15:367–381. xi
36. Derdeyn CP, Shaibani A, Moran CJ, Cross DT 3rd, Grubb RL Jr, Powers WJ. Lack of correlation between pattern of collateralization and misery perfusion in patients with carotid occlusion. Stroke. 1999; 30:1025–1032.
37. Uzunca I, Asil T, Balci K, Utku U. Evaluation of vasomotor reactivity by transcranial Doppler sonography in patients with acute stroke who have symptomatic intracranial and extracranial stenosis. J Ultrasound Med. 2007; 26:179–185.
38. Arandjic D, Bonutti F, Biasizzo E, Ciraj-Bjelac O, Floreani M, Giustizieri M, et al. Radiation doses in cerebral perfusion computed tomography: patient and phantom study. Radiat Prot Dosimetry. 2013; 154:459–464.
39. Zhang D, Cagnon CH, Villablanca JP, McCollough CH, Cody DD, Stevens DM, et al. Peak skin and eye lens radiation dose from brain perfusion CT based on Monte Carlo simulation. AJR Am J Roentgenol. 2012; 198:412–417.
40. Ma J, Zhang H, Gao Y, Huang J, Liang Z, Feng Q, et al. Iterative image reconstruction for cerebral perfusion CT using a pre-contrast scan induced edge-preserving prior. Phys Med Biol. 2012; 57:7519–7542.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download